Spina bifida is a neurologic malformation in which an area of the fetal spine doesn’t develop and close properly early in pregnancy, leaving a section of the spinal cord and spinal nerves exposed. Because of the opening in the spine, the spinal cord and nerves of the spinal cord may become damaged. A spinal cord that is damaged may not be able to send messages to and from the brain regarding body temperature, pain/touch sensations, bodily movements, and other important functions.
Myelomeningocele (MMC) is the most common and most serious form of spina bifida, in which part of the spinal cord and surrounding nerves protrude from the fetus’s back. Usually, the exposed spinal cord and nerves are contained in a sac that is exposed to amniotic fluid in the womb. Continuous bathing of the fragile developing spinal cord to amniotic fluid, being stretched over the cerebrospinal fluid (CSF) filled cyst, and direct trauma by contact with the uterine wall can result in progressive injury and neurologic deficits.
A baby born with meningomyelocele may experience:
- Bladder and bowel problems (incontinence).
- Weakness, loss of sensation or paralysis below level of the defect.
- Arnold-Chiari Malformation type II: A condition that affects how the back of the brain forms, leading to positioning of a portion of the cerebellum near the upper spinal column. This lower-than-normal brain positioning obstructs the flow of cerebrospinal fluid out of the brain, and may lead to hydrocephalus and cognitive impairment.
- Hydrocephalus (water in the brain): Occurs when there’s too much cerebrospinal fluid in the head.
- Abnormal development of the feet and legs, such as clubfoot.
Families at the Fetal Care Center at Connecticut Children’s have treatment options for babies diagnosed with myelomeningocele that include repair in utero or soon after birth by our team of Maternal-Fetal Medicine specialists, Fetal Surgeons, and Pediatric Neurosurgeons.
Would you like to schedule an appointment with our Fetal Care Center?
The diagnosis of spina bifida used to depend upon the detection of elevated alpha-fetoprotein in the amniotic fluid (Allen 1973), or in the maternal serum (MSAFP). Due to improvements in ultrasound technology, over 90% of cases of fetal spina bifida are now identified on routine sonographic screening (Copp 2016). Ultrasound may demonstrate the cystic myelomeningocele spinal defect or detect secondary intracranial signs that are associated with myelomeningocele. Sometimes the fetal position, such as back against the uterine wall, makes it difficult to see the spinal malformation. Especially in these cases, the intracranial abnormalities associated with myelomeningocele can be helpful and include scalloping of the frontal bones known as “lemon sign” or crowding of the cerebellar hemispheres known as the “banana sign”.
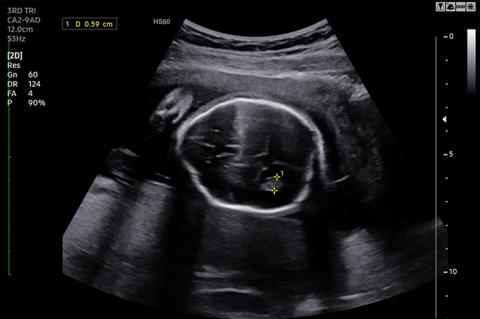
The ultrasound above demonstrates scalloping of the frontal bones which is known as “lemon sign.”
Fetal MRI may be helpful in defining the intracranial anatomy in myelomeningocele demonstrating the features of the Arnold-Chiari malformation type II, which include compression of the fourth ventricle, obliteration of the cerebrospinal fluid spaces around the cerebellum, herniation of the cerebellar tonsils through the foramen magnum, breaking of the tectum vertical orientation of the tentorium, and ventriculomegaly. Ultrasound and fetal MRI can be used to determine the level of the spinal defect, which is defined by the highest point in which the bony spinal elements splayed open.
In some cases, it may be difficult to distinguish spina bifida aperta (open neural defect) from spina bifida occulta (covered neural tube defect). In cases of spina bifida aperta, amniotic fluid levels of alpha-fetoprotein and acetylcholinesterase will be significantly elevated. In contrast, in spina bifida occulta, amniotic fluid levels of alpha-fetoprotein and acetylcholinesterase will be normal.
The mother should be made aware of all options for the care of her baby, including prenatal repair and postnatal repair. The decision to pursue prenatal or postnatal myelomeningocele spina bifida treatment is a very personal one that may be influenced by such factors:
- Gestational age at diagnosis
- The level and extent of the myelomeningocele lesion on the spine
- Maternal health considerations
- Family situation
- Plans for future pregnancies
Because spinal cord damage is progressive during gestation, one option is to treat your baby by performing myelomeningocele repair in utero to help prevent further damage with the goal of giving your baby an improved quality of life.
When a fetus has myelomeningocele or spina bifida aperta, the spinal cord and nerves are exposed to elements that could cause further harm from exposure to amniotic fluid, stretch injury from cerebrospinal fluid distending the cyst and direct trauma. Open fetal surgery may prevent ongoing injury from occurring during the remainder of the pregnancy. Prenatal repair also significantly reduces the need for ventriculoperitoneal shunting to treat hydrocephalus associated with myelomeningocele, but there are potential risks of fetal surgery for both mother and baby. Another option is to have the myelomeningocele repaired after the baby is born. This option has the lowest maternal risks associated with it, but increases the need for ventriculoperitoneal shunting in the baby.
During fetal surgery for myelomeningocele, our surgical team will access the uterus and repair the defect in your baby’s spine. (Dr. Crombleholme explains the surgical interventions for MMC repair in this video.) The spinal cord is released from other tissues and the opening in the spinal canal is closed in layers including the dura, the fascia, and skin from your baby’s back creating a water-tight closure to protect the developing spinal cord and nerves. The fetal myelomeningocele can be repaired either by an open surgical technique or by a fetoscopic surgical technique. The fetoscopic approach reduces the size of the incision in the womb to perform the repair, but has yet to be proven to be equivalent to the open fetal surgical repair in terms of the baby’s outcomes. The uterus and maternal abdomen are surgically closed and the baby is closely monitored until delivery.
If you and your fetal care team decide the best course of action is to repair your baby’s myelomeningocele after birth, you will receive frequent ultrasounds to monitor the growth of your baby throughout the pregnancy. Surgical repair to close the spina bifida will take place within the first few days of your baby’s life.
We work with a multidisciplinary team of Neonatologists, Pediatric Neurosurgeons, Pediatric Urologists, Pediatric Orthopedists, Physical and Occupational Therapists, Developmental Pediatricians, Social Workers, and other specialists as needed to help your baby thrive in our multispecialty Spina Bifida Clinic. All babies with spina bifida aperta, whether they undergo prenatal repair or postnatal repair, are followed in our Spina Bifida Clinic. If you travel from a great distance to have fetal surgery or postnatal surgery, we work with the spina bifida clinic closest to your home to facilitate postnatal follow-up care.
Fetal myelomeningocele repair decreases the likelihood that hydrocephalus will require ventriculoperitoneal shunting after birth. In the management of myelomeningocele study (MOMS), the need for ventriculoperitoneal shunting by 12 months of age was 44% in the prenatal myelomeningocele repair group versus 84% in the postnatal myelomeningocele repair group. Prenatal repair was also found to have a decreased risk of shunt malfunction in those requiring VP shunt at only 13.4% versus 40.2% among postnatally repaired myelomeningoceles.
The advantage to prenatal myelomeningocele repair was maintained through school age with only 49% requiring shunting versus 85% among postnatally repaired myelomeningoceles. Similarly, only 23% of children who had fetal surgery required shunt revision surgery compared to 60% among postnatally repaired myelomeningocele.
The MOMS trial also demonstrated that the development of progressive hydrocephalus requiring treatment did not negatively impact distal motor function. This suggests that the incidence of progressive hydrocephalus requiring ventriculoperitoneal shunting and potential improvement of motor function are independent outcomes.
Motor function following prenatal myelomeningocele repair was found to be at least 2 levels improved between the anatomic level and the functional motor level in 26.4% with prenatal repair versus 11.4% in postnatal repair. This improvement in motor function in prenatally repaired myelomeningocele persists to school age in which 29% were walking independently (vs. 11% with postnatal repair) and 64% ambulating with orthotics or assistive devices (vs. 39% with postnatal repair).
The need for clean intermittent catheterization was no different between prenatal and postnatal groups at 30 months of follow-up. However, by school-age prenatally repaired myelomeningocele showed a decrease in the need for clean intermittent catheterization (61.5% versus 87.2% in postnatal repair). In addition, the prenatal repair group had improved rates of control over voiding and decreased in post-void residuals.
Testing in school-aged children has shown no difference in scores on a composite of mental psychomotor development between prenatal and postnatal repair cohorts. Vineland composite scores (a survey designed to assess adaptive behavior in communication, daily living skills, socialization, motor skills, and maladaptive behavior) in these children were not significantly different between prenatal and postnatal surgery groups. The presence of a ventriculoperitoneal shunt has not been demonstrated to negatively impact cognitive development in children undergoing prenatal repair. The presence of a VP shunt has not been associated with any of the major secondary outcome measures: walking independently, differences in anatomic versus functional motor levels of 2 or more levels better than expected, or composite scoring in mental and psychomotor developmental indices. This is despite the fact that larger ventricles at the time of prenatal repair are associated with the increased need for ventriculoperitoneal shunting. In ventricles less than 10 mm at the time of prenatal repair, the VP shunt rate was only 20%. In cases in which the ventricles were between 10 and 15 mm at the time of prenatal repair, the VP shunt rate was 40%. Lastly, in cases in which ventricles were over 15 mm at the time of prenatal repair, the VP shunt rate was 85%. Prenatally repaired cases of myelomeningocele appear to benefit despite enlarged ventricles and the need for VP shunting.
For Medical Professionals
The incidence and live-birth prevalence of myelomeningocele are correlated strongly with ethnic and geographic factors. The highest frequencies of neural tube defects are found in Great Britain, Ireland, Pakistan, Northern India, Egypt, and Arab countries. The lowest incidences are found in Finland, Japan, and Israel. Even in the United States, a geographic distribution of these defects occurs, with the frequency being the highest in the East and the South, and the lowest in the West (Harmon et al. 1995). There is an increased incidence of neural tube defects in Hispanics, especially if the mother was born in Mexico (Shaw et al. 1994).
The live-birth prevalence of infants with myelomeningocele has changed dramatically since the advent of widespread maternal serum screening for AFP. Before 1980, the live-birth prevalence of infants with neural tube defects was between 1.5 and 4.5 per 1000 live births. After 1980, this decreased from 0.74 to 2.5 per 1000 live births (Shurtleff and Lemire 1995). In the United States, the live-birth prevalence of infants with neural tube defects is 0.41 to 1.43 per 1000. The lowest incidence of neural tube defects is in African blacks, who have a live-birth prevalence of 1 per 10,000 (Shurtleff and Lemire 1995). Because the screening tests detect anencephaly as well as myelomeningocele, and many parents decide to terminate pregnancies affected with the uniformly fatal anencephaly, the incidence of myelomeningocele has increased as a percentage of all cases of neural-tube defects (Rieder 1994).
In a review of factors associated with neural-tube defects in the NIH-sponsored Collaborative Perinatal Project that reviewed the pregnancies of 53,000 pregnant women, Myrianthopoulos and Melnick (1987) demonstrated that maternal diabetes mellitus, heart disease, lung disease, and use of diuretics, antihistamines, or sulfonamides were all associated with an increased risk of neural-tube defects. In addition, women who had a short interval between the end of the previous pregnancy and the current pregnancy had an increased incidence of neural tube defects. Most of the cases reviewed in the Collaborative Perinatal Project demonstrated that the neural tube defect was an isolated malformation. More recently, it has been shown that the use of the anticonvulsants valproic acid and carbamazepine are also associated with neural tube defects. Similarly, maternal history of increased alcohol intake, nutritional deficiencies, and use of the folic acid inhibitor aminopterin are also associated with an increased incidence of neural tube defects. Neural tube defects are also associated with low socioeconomic status, a positive family history, and twinning. In general, females are affected more often than males (Källén et al. 1994).
Prior to the mid-1980s, sonographic diagnosis of myelomeningocele relied on the meticulous scanning of the vertebrae for abnormalities. Using this method, neural tube defects were often missed. More recently, the prenatal sonographic diagnosis of myelomeningocele has been enhanced by the recognition of specific brain abnormalities that generally precede the detection of the spinal lesion (Blumenfeld et al. 1993).
The central nervous system (CNS) abnormalities that have been described in neural-tube defects include cerebral ventriculomegaly, microcephaly, abnormalities of the frontal bone, and obliteration of the cisterna magna with an apparently absent cerebellum or an abnormal concavity of the cerebellar hemispheres. These latter findings have been referred to as the “fruit” signs, which include the “lemon” and the “banana” signs. The lemon sign (Fig. 2) describes a concave or flattened frontal contour of the fetal calvarium rather than a normal convex frontal contour. The banana sign describes the posterior convexity of the cerebellum within the posterior cranial fossa (Nicolaides et al. 1986). The lemon sign has been described in 1% of apparently normal fetuses, whereas the banana sign has not been described in normal fetuses.
The abnormal CNS sonographic findings are a consequence of the Arnold–Chiari type II malformation. In a prospective analysis, Campbell et al. (1987), who studied 436 fetuses at high risk for spina bifida, identified 26 fetuses with an open neural tube defect. Of the 26, 17 (62%) had a small biparietal diameter for gestational age, 9 (35%) had an abnormally small head circumference, and 100% had a positive lemon sign. In addition, 25 of 26 fetuses (96%) had a cerebellar abnormality. Of these, nine had an absent cerebellum, and 16 had a positive banana sign. Only one fetus in the study with an open-neural tube defect had a normal cerebellum. These findings were further defined by Van den Hof et al. (1990), who demonstrated that the CNS abnormalities seen in myelomeningocele evolve with gestation. These authors studied 130 fetuses with open spina bifida and demonstrated a relationship between gestational age and the presence of the lemon and banana signs. A lemon sign was present in 98% of fetuses with open spina bifida at ≤24 weeks of gestation, although this finding was seen in only 13% of fetuses at >24 weeks of gestation. Cerebellar abnormalities were seen in 95% of fetuses at any stage of gestation, although the banana sign was more typical at <24 weeks of gestation, and apparent cerebellar absence was typical of fetuses at >24 weeks of gestation. Because the lemon sign is due to decreased intracranial pressure because of caudal herniation of the hindbrain contents, the lack of the lemon sign may be due to skull maturation. Alternatively, the cerebro-ventriculomegaly that is very common in open spina bifida may compensate for the loss of the brain mass. This may displace the skull bones.
More recently, Ball et al. (1993) demonstrated that the lemon sign is not specific to meningomyelocele. In this report of 23 cases of a positive lemon sign, 12 were associated with an open spina bifida, and 6 were seen in cases of encephalocele. An additional 5 fetuses did not have a neural tube defect, although they had a variety of other abnormalities, including thanatophoric dysplasia, cystic hygroma, and agenesis of the corpus callosum. An additional CNS finding associated with meningomyelocele was effacement of the cisterna magna, which was seen in 19 of 20 fetuses studied with myelomeningocele (Goldstein et al. 1989). In a review of 234 fetuses with open spina bifida diagnosed at <24 weeks of gestation, Watson et al. (1991) demonstrated that all but two fetuses had a least one of the cranial abnormalities described for affected fetuses. They also questioned whether there was a higher positive predictive value for open spina bifida when more than one sign was observed antenatally. These authors also cautioned that the evaluation of motor function in the fetus was not predictive of future neuromuscular status.
In addition to the fruit signs, there are several other sonographic findings in neural tube defects a third of which fall into the Arnold-Chiari type II malformation spectrum (Kupalin 2020). Those specifically associated with Arnold-Chiari type II spectrum include funneling of the posterior fossa, small transcerebellar diameter (< 10th percentile), beaked tectum, and ventriculomegaly. In addition to these findings, other sonographic features are also associated with myelomeningocele which includes small head size, corpus callosum, parenchymal brain abnormalities, pointed occipital horns of the lateral ventricles, thin occipital lobe, interhemispheric cysts with prominent massa intermedia. In addition, D’Addario et al., found that almost all fetuses with myelomeningocele have a small clivus-supraoccipital angle to less than 72 degrees (mean of 65o vs 79o in normal fetuses) indicative of a reduced posterior fossa size (D’Addario 2008). Altered CSF flow by the effacement of the 4th ventricle may be responsible for the common association of cavum veli interpositi (CVI) which appears as an anechoic interhemispheric cyst below the splenium of the corpus callosum (Wong 2009, D’Addario 2009). This abnormality is common in myelomeningocele because the anterior part of the 3rd ventricle cannot dilate due to an adhesion to the massa intermedia which is also prevalent in myelomeningocele (Juranek 2010, Williams 1975).
The evaluation of the fetal spine depends on the visualization of the three ossification centers within the fetal vertebra. The centers of the neural arches should be parallel or converging. In the longitudinal plane, the spine should appear like a “railroad track,” with gradual widening toward the fetal head and tapering toward the sacrum. However, the distal part of the spine may not be ossified in healthy fetuses prior to 22 weeks of gestation (Budorick et al. 1995). Spina bifida can be demonstrated in both the coronal and transverse planes. In the coronal plane, widening of the ossification centers in the neural arch interrupts the normal parallel configuration of the vertebral arches (Fig.3). In the transverse plane, ossification centers in the neural arch either diverge or take on a U-shaped configuration (Fig.4). Scoliosis or kyphosis can also associated with neural-tube defects. Kollias et al. (1992) assessed the sonographic accuracy of the estimation of the spinal level involved in the myelomeningocele. Of 28 cases studied, sonographic and pathologic levels were in agreement in 18 (64%) and within one spinal level in 22 (79%).
Other sonographic findings that may suggest a myelomeningocele include a cystic meningeal sac, which may have a shimmering effect with fetal motion (Fig.5) (Budorick et al. 1995). The sonographer should also examine the fetus’s lower extremities for the possibility of clubbed feet.
The incidence of associated anomalies in meningomyelocele is lower in reports derived from living children versus those in autopsy series. In one series of 181 patients with myelodysplasia, 5 had renal malformations and 3 had congenital heart disease (Kreder et al. 1992).
The adjunctive use of magnetic imaging (MRI) of the fetus has provided additional and complementary information to ultrasound examination alone. The results of at least two studies suggest that fetal MRI is superior to ultrasound examination for prenatal diagnosis of the intracranial abnormalities associated with MMC (Dinh et al 1990; Levine et al, 1999). In a comparison of sonography and MRI, both were equally accurate in the assignment of MMC level (Aaronson et al.,2003). As a practical matter, we primarily rely on ultrasound to determine the spinal level of myelomeningocele. MRI may be a particularly helpful adjunct to ultrasound examination when there is a large maternal body habitus, oligohydramnios, low position of fetal head or posterior position of fetal spine present.
Fetal MRI is also particularly useful in evaluating the posterior fossa in fetuses with myelomeningocele and determining the presence or absence of hindbrain herniation. Fetal MRI is also able to detect the presence of grey matter heterotopia which occurs in up to 20% of cases of myelomeningocele (based on autopsy studies). We consider fetal MRI an invaluable adjunct to ultrasound in the evaluation of the fetus with myelomeningocele. We also use post-operative fetal MRI to evaluate the response to prenatal myelomeningocele repair by routinely obtaining a follow-up MRI two weeks following the surgery to determine if there has been a reversal of hindbrain herniation.
Fetal MRI is most commonly performed on a 1.5 TESLA scanner although 3.0 TESLA scanners are favored in some centers. There have been no proven harmful effects on the developing human fetus from limited exposure to electromagnetic fields occurring during fetal MRI (Schwartz 1982, Thomas 1981, Mirsky 2015). In the United States, fetal MRI is generally not performed during the first trimester of gestation out of a theoretical concern for teratogenesis and most centers do not perform MRI prior until at least 16 weeks’ gestation. The American College of Radiology, however. does not stipulate any special considerations regarding MRI of a fetus at any stage of pregnancy (Kanal st al 2013, Chartier 2013)
Recent studies have demonstrated that detailed fetal brain MRI reveals subtle anomalies with potential clinical significance in a high proportion of fetuses eligible for spina bifida repair (Saenz Cortez 2018). Trigo et al., found that, among fetuses with myelomeningocele who were eligible for fetal surgery, frequently had abnormal corpus callosum and/or gray matter heterotopia on fetal MRI (Trigo 2022) This study also found that sacral myelomeningocele, larger ventricular size, and abnormal cavum septum pellucidum were associated with a higher risk for abnormal corpus callosum and/or gray matter heterotopia. These authors suggested a “third hit” in which hydrocephalus caused by the Chiari Arnold-Chiari type 2 malformation could contribute to the generation of other brain abnormalities such as dysgenesis of the corpus callosum and migrational abnormalities which result in gray matter heterotopia. Several authors have suggested that these subtle abnormalities detected on fetal MRI, including corpus callosum abnormalities, grey matter heterotopia, or larger vermian displacement, could account for less favorable outcomes after fetal MMC repair than would be expected based solely on the anatomic level of the MMC (Khavalah 2021 Richmond 2019 product Brodoefel 2013).
The differential diagnosis for myelomeningocele includes isolated hemivertebrae. The lemon sign, as stated earlier, has also been seen in encephalocele, thanatophoric dysplasia, cystic hygroma, and craniosynostosis. The demonstration of a mass near the fetal sacrum must also call to mind the possible diagnosis of sacrococcygeal teratoma. Sacrococcygeal teratomas are large cystic or solid masses arising from the coccyx. These masses may be associated with fetal hydrops or polyhydramnios. If the fetal sacral bones cannot be visualized, the differential diagnosis also should include caudal regression syndrome and sirenomelia.
The severity of complications observed in children with MMC prompted interest in the potential of in-utero myelomeningocele repair to prevent these complications. The rationale for repair in utero is that the open neural tube defect allows exposure of the spinal cord to secondary injury from exposure to amniotic fluid, direct trauma, or hydrostatic pressure (Adzick and Walsh, 2003). This has been referred to as the “two-hit hypothesis” (Hutchins et al., 1996).
Meuli-Simmen et al. (1995) used the latissimus dorsi muscle flap for fetal myelomeningocele repair in seven sheep fetuses with a surgically created lumbar myelomeningocele. Three fetuses survived the pregnancy. At term, the sheep survivors had healed cutaneous wounds and normal hind-limb function. These authors concluded that the latissimus dorsi flap is suitable for fetal surgery and provides efficient coverage of the lesion.
As a result of experimental work in animals, it is known that the neurologic deficits associated with open spina bifida are due partly to chronic mechanical injury, stretch, and chemical trauma induced by exposure to amniotic fluid. These exposures progressively damage the unprotected fetal neural tissue during gestation. In fetal sheep, in utero repair of neural tube defects restored neurologic function by the time of birth (Meuli et al., 1997).
Open fetal surgery to repair myelomeningocele is performed by maternal laparotomy and hysterotomy. The cystic membrane of the lesion is excised, the placode is released from tethering, the dura is closed over the placode and fascial layers are developed and closed over the defect. Lastly, skin flaps are developed circumferentially to complete the closure of the defect (Adzick et al., 1998). Amniotic fluid is replaced with warmed lactated Ringer’s solution. After repair, tocolysis is maintained with magnesium sulfate infusion, indomethacin rectal suppositories and later converted to oral nifedipine.
The first attempt to repair by providing skin coverage for myelomeningocele was reported by Bruner et al. in 1997, using a maternal split thin skin graft endoscopically applied (Bruner et al., 1997). One patient died shortly after the surgery and the second patient showed no sign of improvement postnatally. Subsequently, the same group reported four patients who underwent open fetal surgical repair between 28 and 32 weeks gestation with reversal of hindbrain herniation at birth (Tulipan and Bruner, 1998).
Similarly, the group at Children’s Hospital of Philadelphia (CHOP) reported the first early gestation success with reversal of hindbrain herniation (Adzick et al., 1998). This was subsequently confirmed in a series of 10 patients undergoing MMC closure at 22 to 25 weeks’ gestation (Sutton et al., 1999), in which 9 of 10 survived with reversal of hindbrain herniation. Four of the 9 later required ventriculoperitoneal shunting (Adzick and Walsh, 2003). Bruner et al. (1997) showed that 62% of 29 patients had a reversal of hindbrain herniation when operated on between 24 and 30 weeks’ gestation. Ventriculoperitoneal shunting was required in 17 of 29 (59%) but still compared favorably with historical controls in which 90% required ventriculoperitoneal shunting (Rintoul et al., 2002).
Prior to the start of the Management of Meningomyelocele Study (MOMS) trial, experience with open fetal surgical repair had been performed at CHOP, the University of California at San Francisco, the University of North Carolina, and Vanderbilt with a combined experience of approximately 160 patients. Findings suggested improved outcomes compared to historical controls. Sutton et al. reported that hindbrain herniation was uniformly reversed in the CHOP experience and only 43% required ventriculoperitoneal compared to an 84% rate observed in 297 historical controls (Sutton et al., 1999; Rintoul et al., 2002). Of note in this series of 50 patients were 3 deaths from complications from premature delivery at 25 weeks. The average gestational age at delivery was 34 4/7 weeks (Rintoul et al., 2002). While this study suggested a reduced need for ventriculoperitoneal shunting, it should be pointed out that the controls were historical, and neurosurgical indications for shunting had become more conservative during this period. In addition, some infants undergoing fetal surgery for myelomeningocele merely experienced delayed time for ventriculoperitoneal shunting.
Danzer et al. (2007) have reported that open fetal surgery for myelomeningocele alters fetal head growth. Repaired myelomeningocele fetuses have disproportionately small head circumference measurements while the lateral ventricles progressively enlarge (Van den Hof et al., 1990; Babcock et al., 1994; Bannister et al., 1998). In a series of 50 fetuses undergoing open fetal surgery to repair myelomeningocele, Danzer et al. found a significant increase in the cortical index (head circumference/lateral ventricular diameter). Early neurodevelopmental evaluations at 2 years of age in the cohort of 51 myelomeningocele patients treated by open fetal surgery at CHOP reveal that 67% had cognitive language and personal-social skills in the normal range, 20% had mild delays and 13% had significant delays (Johnson et al., 2006).
The lower extremity neuromotor evaluation following open fetal surgery for myelomeningocele suggests that 58% of patients had a better than predicted lower extremity function compared to infants with postnatally repaired myelomeningocele (Danzer et al., 2006; Carr, 2007). In this relatively early follow-up series (39 + 15 months) of open fetal surgically repaired MMC, 21 children (52.5%) walked independently, 8 (20%) walked with braces, 7 (15.5%) ambulated with a walker and 4 (10%) used a wheelchair. This was in contrast to less favorable outcomes in postnatally repaired myelomeningocele in which only 1 child (6%) walked independently, 5 (29%) walked with braces, 10 (58.8%) ambulated with a walker and 1 (6%) used a wheelchair (follow-up at 41.9 ± 16.6 months). This early assessment of lower extremity function may be misleading, as many children who had previously been able to ambulate with or without braces or walkers revert to a wheelchair at puberty due to increased weight and size that make ambulation very difficult.
Carr (2007) reported his experience with urodynamic evaluation of 22 patients who underwent fetal surgical repair of myelomeningocele at CHOP. In 13 of 22 patients, he evaluated voiding spontaneously with 3 of 22 (13.6%) achieving volitional voiding. This compares favorably with the expected 2% to 3% volitional voiding in postnatal myelomeningocele repair (Carr, 2006). The remainder of the 22 patients had either vesicoureteral reflex (10%), urinary tract infections (33%), required vesicostomy (5%), or clean intermittent catheterization.
In order to address many of the questions raised by early outcomes of open fetal surgery in myelomeningocele, the NIH funded the MOMS trial. This prospective randomized trial compared outcomes with open fetal surgery performed at 18 to 25 6/7 weeks’ gestation with postnatal surgery.
Critical Assessment of the MOMS Trial
The MOMS trial was stopped after recruitment of 185 of a planned 200 subjects when a significant difference was observed in the primary endpoint of the study was reached by 158 of the 185 subjects (see Table 1). The primary outcome of the MOMS trial was the composite outcome of fetal or neonatal death or the need for a ventriculoperitoneal shunt at 12 months of age. This occurred in 68% of infants in the prenatal surgery group, but 98% of the postnatal surgery group (p<0.001) (see Table 2). Consistent with this finding, the incidence of infants who had no evidence of hindbrain herniation in the prenatal cohort was 36% versus only 4% in the postnatal surgery group. In addition, the prenatal surgery group had a lower rate of moderate or severe hindbrain herniation (25%) compared to the postnatal surgery group (67%). Although the rate of epidermical cysts was similar in both groups, the incidence of cord tethering requiring subsequent surgical release, was significantly higher in the prenatal surgery group (8% vs 1%). In contrast, the postnatal surgery group required more Chiari decompression surgery (4/80 5%) versus prenatal surgery group (1/77 1%) and had a higher incidence of brainstem kinking (moderate or severe 14% with prenatal surgery and 37% with postnatal surgery). The incidence of syringomyelia was 39% in the prenatal surgery and 58% in the postnatal surgery groups.
Table 1. Baseline Characteristics of the Study Population*
| Characteristic | Prenatal Surgery (N=78) | Postnatal Surgery (N=80) |
|---|---|---|
| Fetal sex female – no (%) | 35 (45) | 51 (64) |
| Gestational age at randomization – wk | 23.6±1.4 | 23.9±1.3 |
| Maternal age at screening – yr | 29.3±5.3 | 28.8±4.9 |
| Race or ethnic group – no. (%)† | ||
| -White | 73 (94) | 74 (92) |
| -Black | 1 (1) | 1 (1) |
| -Hispanic | 2 (3) | 4 (5) |
| -Other | 2 (3) | 1 (1) |
| Married or living with partner – no. (%) | 73 (94) | 74 (92) |
| Years of schooling – no. | 14.8±1.7 | 15.0±1.6 |
| Body-mass index at trial entry‡ | 26.2±3.7 | 25.9±3.9 |
| Current smoker – no. (%) | 6 (8) | 4 (5) |
| Either parent with familial history of neural-tube defect – no. (%) | 8 (10) | 14 (18) |
| Nullipara – no. (%) | 33 (42) | 36 (45) |
| Previous uterine surgery – no. (%) | 11 (14) | 8 (10) |
| Cervical length – mm | 38.9±7.3 | 39.7±5.7 |
| Anterior placenta – no. (%) | 36 (46) | 32 (40) |
| Lesion level on ultrasonography – no. (%) | ||
| -Thoracic | 4 (5) | 3 (4) |
| -L1-L2 | 21 (27) | 10 (12) |
| -L3-L4 | 30 (38) | 45 (56) |
| -L5-S1 | 23 (29) | 22 (28) |
| Lesion level L3 or lower on ultrasonography – no. (%) | 53 (68) | 67 (84) |
| Club foot on ultrasonography – no. (%) | 20 (26) | 15 (19) |
*Plus-minus values are means ±SD. The only between-group comparisons that were significant were the female se of the fetus and a lesion level of L3 or lower on ultrasonography (P=0.02 for both comparisons). Percentages may not total 100 because of rounding. †Race or ethnic group was self-reported.
‡ The body-mass index is weight in kilograms divided by the square in height in meters. Adapted from Adzick et al A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele.
From Adzick NS, Thom EA, Spong KY et al: A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364: 993-1004, 2011
Table 2. Infant Outcomes at 12 Months*
| Outcome | Prenatal Surgery (N=78) | Postnatal Surgery (N=80) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Primary Outcome-no. (%) | 53 (68) | 78 (98) | 0.70 (0.58-0.84)† | <0.001 |
| Components of primary outcome – no. (%) | <0.001 | |||
| Death before placement of shunt | 2 (3) | 0 | ||
| Shunt criteria met | 51 (65) | 74 (92) | ||
| Shunt placed without meeting criteria | 0 | 4 (5) | ||
| Placement of shunt – no. (%) | 31 (40) | 66 (82) | 0.48 (0.36-0.64) | <0.001 |
| Any hindbrain herniation – no./total no. (%) | 45/70 (64) | 66/69 (96) | 0.67 (0.56-0.81) | <0.001 |
| Degree of hindbrain herniation – no./total no. (%) | <0.001‡ | |||
| None | 25/70 (36) | 3/69 (4) | ||
| Mild | 28/70 (40) | 20/69 (29) | ||
| Moderate | 13/70 (19) | 31/69 (45) | ||
| Severe | 4/70 (6) | 15/69 (22) | ||
| Any brainstem kinking – no./total no. (%) | 14/70 (20 | 33/69 (48) | 0.42 (0.25-0.71) | <0.001 |
| Degree of brainstem kinking – no./total no. (%) | 0.001‡ | |||
| None | 56/70 (80) | 36/69 (52) | ||
| Mild | 4/70 (6) | 8/69 (12) | ||
| Moderate | 7/70 (10) | 17/69 (25) | ||
| Severe | 3/70 (4) | 8/69 (12) | ||
| Abnormal location of fourth ventricle – no./total no. (%) | 32/70 (46) | 49/68 (72) | 0.63 (0.47-0.85) | 0.002 |
| Location of fourth ventricle – no./total no. (%) | <0.001‡ | |||
| Normal | 38/70 (54) | 19/68 (28) | ||
| Low | 28/70 (4) | 29/68 (43) | ||
| At foramen magnum | 1/70 (1) | 8/68 (12) | ||
| Below foramen magnum | 3/70 (4) | 12/68 (18) | ||
| Syringomyelia – no./total no. (%) | 27/69 (39) | 39/67 (58) | 0.67 (0.47-0.96) | 0.03 |
| Epidermoid cyst – no./total no. (%) | 2/67 (3) | 1/66 (2) | 1.97 (0.18-21.20) | 1 |
| Epidermoid cyst – no./total no. (%) | 2/67 (3) | 1/66 (2) | 1.97 (0.18-21.20) | 1 |
| Surgery for tethered cord – no./total no. (%) | 6/77 (8) | 1/80 (1) | 6.15 (0.76-50.00) | 0.06 |
| Chiari decompression surgery – no./total no. (%) | 1/77 (1) | 4/80 (5) | 0.26 (0.03-2.24) | 0.37 |
| Shunt infection – no./total no. (%) | 5/77 (6) | 7/80 (9) | 0.73 (0.24-2.21) | 0.58 |
*Percentages may not total 100 because of rounding.
†The relative risk for the composite primary outcome is reported with a 97.7% confidence interval.
‡The between-group comparison was performed with the use of the Cochran-Armitage test for trend.
From Adzick NS, Thom EA, Spong KY et al: A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364: 993-1004, 2011
While most studies have only a single primary endpoint, the MOMS trial had a second primary endpoint, a score derived from a composite of the Bayley Mental Developmental Index and the difference between the functional level and the anatomical level at 30 months of age. (Table 3)
This composite of the Bayley Mental Developmental Index and difference between the functional and anatomic level of the lesion was significantly better in the prenatal surgery group. However, when analyzed separately, there was no difference between the groups in the Bayley Mental Developmental Index. The prenatal surgery group did significantly better in the difference between motor function and anatomical level (p=0.001). This must be interpreted cautiously as the differences between anatomic and functional levels have been reported previously in MMC.
Similarly, while there is a significantly higher incidence of children able to walk, with or without orthotics, in the prenatal surgery group, may not be a sustainable outcome. It is known that many more children will be ambulatory, with or without orthotics, as toddlers only to become wheelchair bound as teenagers as their body mass increases and the work of walking becomes excessive.
While the primary outcomes of the MOMS trial are encouraging, these results were achieved at significant maternal and fetal risk in the prenatal surgery group. (Table 4)
Table 3. Outcomes of Children at 30 months*
| Outcome | Prenatal Surgery (N=64) | Postnatal Surgery (N=70) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcome score | 148.6±57.5 | 122.6±57.2 | 0.007 | |
| Primary outcome components | ||||
| Bayley Mental Development Index† | 89.7±14.0 | 87.3±8.4 | 0.53 | |
| Difference between motor function and anatomical levels‡ | 0.58±1.94 | -0.69±1.99 | 0.001 | |
| Bayley Mental Development Index – no./total no. (%)† | ||||
| ≥50 | 60/62 (97) | 59.67 (88) | 1.10 (1.00-1.21) | 0.1 |
| ≥85 | 46/62 (74) | 45/67 (67) | 1.10 (0.88-1.38) | 0.38 |
| Difference between motor function and anatomical levels | 0.002§ | |||
| No./Total No. (%)‡ | ||||
| ≥Two levels better | 20/62 (32) | 8/67 (12) | ||
| One level better | 7/62 (11) | 6/67 (9) | ||
| No difference | 14/62 (23) | 17/67 (25) | ||
| One level worse | 13/62 (21) | 17/67 (25) | ||
| ≥Two levels worse | 8/62 (13) | 19/67 (28) | ||
| Bayley Psychomotor Development Index † | ||||
| Mean | 64.0±17.4 | 58.3±14.8 | 0.03 | |
| ≥50 – no./total no. (%) | 29/62 (47) | 23/67 (34) | 1.36 (0.89-2.08) | 0.15 |
| ≥85 – no./total no. (%) | 10/62 (16) | 4/67 (6) | 2.70 (0.89-8.17) | 0.06 |
| Peabody Development Motor Scales ¶ | ||||
| Stationary score | 7.4±1.1 | 7.0±1.2 | 0.03 | |
| Locomotion score | 3.0±1.8 | 2.1±1.5 | 0.001 | |
| Object manipulation score | 5.1±2.6 | 3.7±2.1 | <0.001 | |
| Walking independently on examination – no./total no. (%) | 26/62 (42) | 14/67 (21) | 2.01 (1.16-3.48) | 0.01 |
| Walking status – no./total no. (%) | 0.03 | |||
| None | 18/62 (29) | 29/67 (43) | ||
| Walking with orthotics or devices | 18/62 (29) | 24/67 (36) | ||
| Walking without orthotics | 26/62 (42) | 14/67 (21) | ||
| WeeFIM score║ | ||||
| Self-care | 20.5±4.2 | 19.0±4.2 | 0.02 | |
| Mobility | 19.9±6.4 | 16.5±5.9 | 0.003 | |
| Cognitive | 23.9±5.2 | 24.1±5.9 | 0.67 |
*Plus-minus values are means ±SD. Listed are data for 134 of 136 patients who underwent randomization before December 1, 2007; data for 2 patients were not available. Before 30 months, there were 5 deaths (2 in the prenatal-surgery group and 3 in the postnatal-surgery group), so data for those infants are not included in any category except the primary-outcome score. Percentages may not total 100 because of rounding.
†On the Bayley Scales of Infant Development II, the Mental Development Index and the Psychomotor Development Index are both scaled to have a population mean (±SD) of 100±15, with a minimum score of 50 and a maximum score of 150. Higher scores indicate better performance.
‡For the difference between the motor-function level and the anatomical level, positive values indicate function that is better than expected on the basis of the anatomical level.
§The between-group comparison was performed with the use of Cochran-Armitage test for trend.
¶On the Peabody Developmental Motor Scales, the mean (±SD) score was 10±3, with a minimum score of 0 and a maximum score of 20. Higher scores indicate better performance.
║On the WeeFIM evaluation, the score on the self-care measurement ranges from 8 to 56, and scores on the mobility and cognitive measurements range from 5 to 35, with higher scores indicating greater independence. Adapted from Adzick et al A
From Adzick NS, Thom EA, Spong KY et al: A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364: 993-1004, 201.
Table 4. Maternal and Fetal or Neonatal Outcomes*
| Outcome | Prenatal Surgery (N=78) | Postnatal Surgery (N=80) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| Maternal Outcome | ||||
| Chorioamniotic membrane separation – no. (%) | 20 (26) | 0 | NA | <0.001 |
| Pulmonary edema – no. (%) | 5 (6) | 0 | NA | 0.03 |
| Modified biophysical profile <8 – no. (%) † | 13 (17) | 6 (8) | 2.22 (0.89-5.55) | 0.08 |
| Oligohydramnios – no. (%) | 16 (21) | 3 (4) | 5.47 (1.66-18.04) | 0.001 |
| Placental abruption – no. (%) | 5 (6) | 0 | NA | 0.03 |
| Gestational diabetes – no. (%) | 4 (5) | 5 (6) | 0.82 (0.23-2.94) | 1 |
| Chorioamnionitis – no. (%) | 2 (3) | 0 | NA | 0.24 |
| Preeclampsia or gestational hypertension – no. (%) | 3 (4) | 0 | NA | 0.12 |
| Spontaneous membrane rupture – no. (%) | 36 (46) | 6 (8) | 6.15 (2.75-13.78) | <0.001 |
| Spontaneous labor – no. (%) | 30 (38) | 11 (14) | 2.80 (1.51-51.8) | <0.001 |
| Blood transfusion at delivery – no. (%) | 7 (9) | 1 (1) | 7.18 (0.90-57.01) | 0.03 |
| Status of hysterotomy site at delivery-no./total no. (%) | ||||
| Intact, well-healed | 49/76 (64) | |||
| Very thin | 19/76 (25) | |||
| Area of dehiscence | 7/76 (9) | |||
| Complete dehiscence | 1/76 (1) | |||
| Fetal or neonatal outcome | ||||
| Bradycardia during fetal or neonatal repair – no. (%) | 8 (10) | 0 | NA | 0.003 |
| Perinatal death – no. (%) | 2 (3) | 2 (2) | 1.03 (0.14-7.10) | 1 |
| Gestational age at birth – wk | 34.1±3.1 | 37.3±1.1 | <0.001 | |
| Gestational age at birth – no. (%) | <0.001‡ | |||
| <30 wk | 10 (13) | 0 | ||
| 30-34 wk | 26 (33) | 4 (5) | ||
| 35-36 wk | 26 (33) | 8 (10) | ||
| ≥37 wk | 16 (21) | 68 (85) | ||
| Birth Weight | ||||
| Mean – g | 2383±688 | 3039±469 | <0.001 | |
| Less than 10th percentile – no. (%) | 3 (4) | 7 (9) | 0.45 (0.12-1.66) | 0.33 |
| Dehiscence at repair site – no./total no. (%) | 10/77 (13) | 5/80 (6) | 2.05 (0.73-5.73) | 0.16 |
| Apnea – no./total no. (%) | 28/77 (36) | 18/80 (22) | 1.62 (0.98-2.67) | 0.06 |
| Pneumothorax – no.total no. (%) | 1/77 (1) | 1/80 (1) | 1.05 (0.07-16.53) | 1 |
| Respiratory distress syndrome – no./total no. (%) § | 16/77 (21) | 5/80 (6) | 3.32 (1.28-8.63) | 0.008 |
| Patent ductus arteriosus-no./total no.(%)¶ | 3/77 (4) | 0 | NA | 0.12 |
| Sepsis-no./total no.(%) ║ | 4/77 (5) | 1/80 (1) | 4.16 (0.48-36.36) | 0.2 |
| Necrotizing enterocolitis-no./total no.(%)** | 1/77 (1) | 0 | NA | 0.49 |
| Periventricular leukomalacia-no./total no.(%) | 4.77 (5) | 2/80 (2) | 2.08 (0.39-11.02) | 0.44 |
| Foot deformity-no./total no.(%) | 39/78 (50) | 36/80 (45) | 1.11 (0.80-1.54) | 0.53 |
* Plus-minus values are means ±SD. There were no instances of bronchopulmonary dysplasia, pulmonary interstitial emphysema, retinopathy of prematurity, pulmonary hypoplasia, grade 3 or 4 intraventricular hemorrhage, or confirmed seizures in either group. Data for neonatal outcomes are listed for 77 infants in the prenatal-surgery group, since 1 infant was stillborn. Additional rare adverse events are provided in Supplementary Appendix, along with the adverse events for 25 additional randomized patients and their offspring (median follow-up from randomization, 29.9 weeks) who underwent randomization on or after July 1, 2009. Percentages may not total 100 because of rounding. NA denotes not applicable.
† The modified biophysical profile is a test of fetal well-being that is calculated on the basis of results of ultrasonography evaluating presence of fetal breathing, movement, and tone, along with the amniotic fluid index. The highest possible score is 8.
‡ The between-group comparison was performed with the use of Cochran-Armitage test for trend.
§Respiratory distress syndrome was defined as a clinical diagnosis of the respiratory distress syndrome type 1 and the need for oxygen therapy (fraction of inspired oxygen, ≥0.40) at 24 hours of age or more.
¶ Patent ductus arteriosus was reported if the infant was treated with medications or surgery.
║Sepsis was defined as confirmation on blood culture, confirmed urinary tract infection, meningitis, or pneumonia.
** Necrotizing enterocolitis was defined as a confirmed clinical diagnosis with any of the following findings observed on radiography, at the time of surgery, or at autopsy: unequivocal presence of intramural air, perforation, erythema and induration of the abdominal wall, intraabdominal abscess formation, or the formation of a stricture after an episode of suspected necrotizing enterocolitis. Adapted from Adzick et al A
From Adzick NS, Thom EA, Spong KY et al: A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364: 993-1004, 2011.
It is important for any mother considering open fetal surgery to repair myelomeningocele that she understands the risks to her with the current pregnancy and all future pregnancies. Mothers in the MOMS trial who had prenatal surgery experienced significantly greater rate of obstetrical complication than noted in the postnatal surgery group including chorioamniotic separation (26% vs 0%, p<0.001) pulmonary edema (6% vs 0%, p=0.03), oligohydramnios (21% vs 4% p=0.001), placental abruption (6% vs 0%, p=0.03), spontaneous rupture of membranes (46% vs 8%, p<0.001), spontaneous labor (38% vs 14%, p>0.001), required blood transfusion at delivery (9% vs 1%, p=0.03), and a hysterotomy scar that was very thin, partially dehisced or completely dehisced in 35% of prenatal surgery cases but was not observed in postnatal cases. The latter is a potential problem for all future pregnancies as the weakened area in the uterus may rupture with labor and requires that all future pregnancies be delivered by cesarean section before the onset of labor.
The fetus with myelomeningocele undergoing fetal surgery to repair the MMC derives direct benefit from the procedure making the potential risks and complications acceptable to assume in the effort to benefit from the surgery. MMC is not ordinarily a lethal condition and would not be expected to result in intrauterine fetal demise or stillbirth (Table 4).
There are postnatal deaths associated with MMC which are most often due to cranial nerve dysfunction thought to be caused by hindbrain herniation resulting in apnea, swallowing difficulties, and bradycardia. While decompression surgery may be beneficial it is not always the case and the two neonatal deaths in the postnatal surgery group of the MOMS trial were due to this complication. More often deaths associated with MMC relate to ventriculoperitoneal shunt infections or shunt malfunction. In years past, renal failure and renal sepsis were common causes of morbidity and mortality in MMC but modern approaches to MMC management utilizing clean intermittent bladder catheterization, are now rarely observed.
There were two fetal deaths in the prenatal surgery group due to intrauterine fetal demise at 26 weeks and neonatal death due to prematurity when the mother delivered at 23 weeks gestation.
Prematurity is an important complication, in all cases of open fetal surgery with the average gestation age at delivery in the prenatal surgery group being 34.1 weeks while the postnatal surgery group delivered on average at 37.5 weeks. But in the prenatal surgery group 13% delivered prior to 30 weeks gestation versus 0% in the postnatal group and 46% delivered ≤ 34 weeks in the prenatal surgery group as compared to 5% in the postnatal surgery group. This much higher incidence of prematurity in the prenatal surgery group likely accounts for the smaller birth weight (2383 ± 688 versus 3039 ± 465 grams p<0.001), greater incidence of apnea (36% versus 22% p<0.06), and the greater incidence of respiratory distress syndrome (21% versus 6% p=0.008).
Surgical Interventions
Under a combination of general and epidural anesthesia, the uterus is exposed via a low transverse laparotomy or a vertical midline incision. The fetus is manually positioned within the planned hysterotomy under ultrasound guidance. The hysterotomy is created a minimum of two fingerbreaths from the placental edge to avoid placental injury during uterine entry and hysterotomy closure. In MOMS trial, the hysterotomy was created using uterine stapling device loaded with absorbable polyglycolic acid staples (Covidien Auto Suture, Norwalk CT).
The use of absorbable polyglycolic acid uterine stapling device is preferred as it is easy to use and it allows quicker and bloodless hysterotomy creation compared to other techniques. We have found that for thicker myometrium, first compressing the myometrium using atraumatic clamps, the stapler works more efficiently. Care is taken not to injure the fetus or cord during uterine entry and the hysterotomy should be as small as possible to allow adequate exposure of the spinal defect for repair. Hemostatic hysterotomy can be created using techniques other than the uterine stapling device. Devices that have been utilized include Harmonic scalpel, tissue sealer (e.g. ENSEAL®, LigaSureTM) monopolar or bipolar coagulation, or simply clamp-and-cut followed by placement of hemostatic sutures. Once exposed, the fetus is given an intramuscular injection of fentanyl (20 mcg/kg), atropine (20 mg/kg) and vecuronium (0.1 mg/kg) before the MMC is repaired.
We monitor the fetal cardiac function and Dopplers continuously in the beginning of the procedure, and intermittently (every few minutes) if the cardiac function and Dopplers remain normal and stable. The uterine and amniotic fluid volume is maintained with warmed Ringer’s lactate infused at body temperature by a Level I Rapid Infusor during the procedure.
Open fetal MMC repair with watertight multi-layer closure is the gold standard for prenatal repair. Although various surgical techniques have been utilized, the fetal MMC repair is usually performed under magnification using surgical loupes or an operative microscope and it includes several key steps: 1) skin incision surrounding the neural tube defect, 2) excision of the sac, 3) releasing of the placode and the rostral spinal cord completely from sac and epidermal tissue, 4) primary closure of the dura using monofilament absorbable suture or closure with dural substitute, 5) myofascial flap mobilization and closure over the dural repair (steps 4 and 5 are often combined as the surface of the fascial flap is covered by dura) 6) skin defect closure primarily if feasible; or with skin substitute for a large skin defect. If myofascial flap is not performed, we augment the primary dural closure using a single layer of dural substitute a layer of NeOx to prevent adhesion of the placode to the dural/myofascial closure. When primary skin closure is not possible, we prefer closing the large skin defect using AlloDerm (Allergan, Madison, NJ, USA) as skin substitute. Direct contact of skin substitute with the dural closure or the placode should be avoided as significant cord tethering had been reported.
We use a layer of DuraGen (Integra Lifesciences, Princeton, NJ) between the dural/myofascial closure and the skin or AlloDerm closure. Others have reported using unilateral or bilateral relaxing flank incisions to facilitate primary skin closure in the midline over the neural tube defect. It is recommended that the epidermal tissue be completely cleaned off the placode and neural tissues to minimize the risk of postoperative epidermoid and dermoid inclusion cyst development. Although it has been reported, re-neurulation of the placode is rarely done during prenatal myelomeningocele repair as the fetal meningeal tissues do not hold sutures well.
Following completion of the MMC repair, the amniotic fluid volume is normalized using warmed Ringer’s lactate, and intra-amniotic antibiotics (nafcillin, vancomycin or clindamycin) are administered while the hysterotomy is closed in layers using monofilament absorbable sutures to incorporate the uterine staples, if used, and amniotic membranes. Crombleholme has reported the use of a third layer to the closure by imbricating the seromuscular layer over the 2-layer hysterotomy closure.
This technique has reduced the rate of hysterotomy complications from 35% to 4% (Zaretsky 2017). It is important to tack the free edges of amniotic membrane up against the uterine wall to minimize risk of chorioamniotic separation and to avoid umbilical cord and fetal injury during hysterotomy closure. Omental patch has been employed by a few groups to reinforce the hysterotomy closure and minimize the risk of amniotic fluid leak. Maternal laparotomy is then closed in layers in standard fashion.
The first attempt to repair a human fetal MMC endoscopically was in April 1994. Subsequently a series of 4 patients (between April 1994 and February 1997) that underwent endoscopic fetal MMC repair using a combination of split-thickness maternal skin graft, Surgicel Absorbable Hemostat, fibrin glue was reported in 1999. Unfortunately, 2 of the fetuses died and the other two required neonatal closure and ventriculoperitoneal shunting without demonstrable benefit from the prenatal repair (Bruner 1999). Thus, the endoscopic procedure was quickly abandoned in favor of the open fetal repair approach. However, open fetal repair through a hysterotomy is associated with higher rates of maternal morbidity and prematurity. The combined favorable outcomes reported by the MOMS trial and the enthusiasm for a less invasive prenatal myelomeningocele repair surgical approach to reduce maternal morbidity and obstetrical complications was re-energized. Fetoscopic myelomeningocele repair is still evolving and the technique is slowly being optimized while its efficacy is being studied.
In general, there are two main fetoscopic approaches: 1) a fully percutaneous fetoscopic MMC repair; and 2) fetoscopic MMC repair via maternal laparotomy and exteriorization of the uterus. In either approach, 2 to 4 trocars of various sizes (6 Fr, 10 Fr, 12 Fr, 14 Fr, 16 Fr, or 5 mm) are placed into the amniotic cavity under partial amniotic carbon dioxide (CO2) insufflation. Warmed, humidified CO2 is preferred over dry, and non-heated CO2 as it reduces the rate of PPROM. Maternal and fetal anesthesia and perioperative management are similar to that of open fetal repair. However, due to the CO2 insufflation of the amniotic cavity fetal echocardiographic monitoring is not possible. Like open repair, the goal of the fetoscopic MMC repair is a watertight closure.
Unfortunately, during the early series of fetoscopic MMC repair, there was a high rate of incomplete closure of the neural tube defect, CSF leakage, fetal surgical site dehiscence, requiring postnatal myelomeningocele re-closure, regardless of the approaches (3-7). When examined carefully, these complications are likely related to technical errors and technical difficulties. These early fetoscopic series also reported increased incidence of premature rupture of membranes and preterm birth. The rates are especially high in the fully percutaneous approach with 91% of premature rupture of membranes and 96% of preterm birth.
A main difference between the fully percutaneous versus the uterine exteriorization approach is the feasibility of trocar or port sites closure in the latter approach. Fetoscopic repair via maternal laparotomy with closure of the trocar sites has a lower preterm prelabor rupture of membranes rate of 28% and lower preterm birth rate of 38%. Recently, Kohn et al. reported 50% vaginal deliveries at a median gestational age of 38 1/7 weeks and 37 1/7 weeks’ gestation with cesarean delivery with no cases of uterine rupture or dehiscence in their 34 patients that had fetoscopic myelomeningocele repair via maternal laparotomy and uterine exteriorization approach (Kohn et al 2018).
Belfort et al. had recently demonstrated that a three-layer fetoscopic MMC closure utilizing a bovine collagen patch as dural substitute, dural/fascial flaps, followed by skin closure, resulted in an improved rates of CSF leakage (0% vs 25%) (Belfort 2020) In addition, this fetoscopic approach showed a much improved rate of reversal of hindbrain herniation at 6 weeks postoperatively (93% vs 60%) when compared to single-layer MMC closure. We also experience no CSF leakage in all of our fetoscopically repaired patients. The Cincinnati group utilize maternal laparotomy, uterine exteriorization, partial amniotic warmed and humidified CO2 insufflation, three trocars, 2-mm and 3-mm instruments, multi-layer closure using double-layer dural substitutes (e.g. Neox cryopreserved umbilical cord allograft, TissueTech, Miami, FL, USA), primary skin closure or AlloDerm skin closure (Patel 2020). Although no case had to be converted from fetoscopic to open fetal surgery, all patients are counseled about the potential need to do so if we could not achieve a satisfactory watertight closure fetoscopically.
A systematic review and meta-analysis performed by Kabagambe et al. showed no significant differences in the primary and secondary outcomes between open fetal repair and fetoscopic repair, and the rate of uterine dehiscence was higher after open repair (Kabagambe 2018). The first annual meeting of the International Fetoscopic Myelomeningocele Repair Consortium was held, and its proceedings were presented (Sanz-Cortez 2019). All participating centers committed to reporting their data to an International Registry in a transparent and standardized manner to facilitate critical analysis and peer review of the collective data with the objectives to optimizing fetoscopic techniques, minimizing maternal morbidity and fetal complications, and improving the outcomes.
Every mother should have a full and complete understanding of all of the potential risks and complications that she would expose herself and her baby to in order to have prenatal surgery to repair MMC. The mother derives no direct benefit from this surgery. The most analogous situation to prenatal surgery for MMC is a parent undergoing living-related kidney donation for transplantation into their child. In the case of prenatal surgery for MMC, the observed risks are mostly obstetrical in nature.
However, one must also consider the potential risks of general anesthesia, deep venous thrombosis, pulmonary embolism, amniotic fluid embolism, massive hemorrhage from abruption requiring hysterectomy and/or death. None of these complications occurred in the MOMS trial but only 92 women were randomized to the prenatal surgery group. It is entirely possible that as more mothers undergo prenatal surgery, these serious obstetrical complications may be observed. The results of the MOMS trial present another management option for mothers to consider for their baby with MMC. It is by no means the only option nor necessarily the preferred option, but an option with considerable attendant risks for both the mother and her baby.
Connecticut Children’s Fetal Care Center, which has one of the most experienced fetal surgeons in the world, is now offering prenatal surgery to repair myelomeningocele. The fetal surgery team includes experts with considerable experience with prenatal surgery for myelomeningocele.
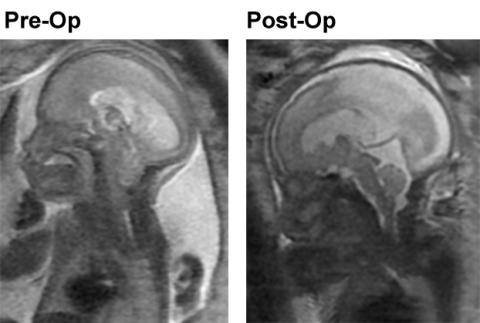
The fetal MRI image above left is the pre-op fetal MRI showing the loss of CSF around the brain, the effacement of the cisterna magna and the 4th ventricle and grade 3 hindbrain herniation of the Chiari II malformation. The fetal MRI image above right is the post-op image demonstrating restoration of the CSF spaces around the brain, the reversal of hindbrain herniation and the return of cisterna magna and the 4th ventricle with evident eburnation of the inner table of the cranial vault at the site of hindbrain herniation now filled with CSF.
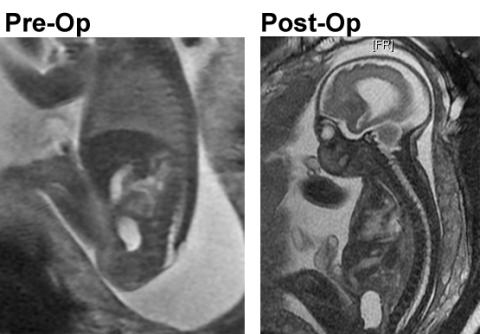
The fetal MRI image above left shows the fetus in the sagittal plane demonstrating the open neural tube defect. The fetal MRI on the right is the post-op image demonstrating the complete repair and the skin and Alloderm coverage of the spinal canal.
Timothy M. Crombleholme, MD, Director of the Fetal Care Center performed 52 prenatal myelomeningocele repairs prior to the MOMS trial, co-authored the first successful early gestation report of prenatal myelomeningocele repair in Lancet as well as the report in JAMA demonstrating reversal of hindbrain herniation with prenatal myelomeningocele repair (Adzick et al 1998, Sutton et al 1999). He was a co-investigator on the MOMS Trial before moving from CHOP to Cincinnati to found The Fetal Care Center of Cincinnati in 2004. He participated in the design and development of the MOMS Trial and performed over 150 open fetal surgeries for the repair of MMC.
In the initial MOMS trial report results were published before all patients randomized could be fully assessed for the primary endpoint (Adzick et al 2011). In a follow-up report Tulipan et al, described the results for the entire cohort which included an analysis of factors that impacted the primary composite endpoint of death or need for ventriculoperitoneal shunting by one year of age.
Logistic regression analysis adjusting for sex and lesion level revealed that ventricular size at screening and gestational age at randomization was associated with a differential effect of prenatal surgery on shunt placentment (Tulipan et al 2015). In the prenatal surgery group, 20% of those with ventricle size < 10 mm, 45.2% with ventricle size 10 to 15 mm, and 79% with ventricle size >15 mm received a ventriculoperitoneal shunt. In contrast, the postnatal group 79.4%, 86%, and 87.5%, respectively, required a shunt (Tulipan et al 2015).
Even though a fetus with ventricles >15 mm will not have any reduction in the need for ventriculoperitoneal shunt there may be other benefits including urologic function with reduced need for chronic intermittent bladder catheterization (Carr et al 2015), reduced rate of shunt complications if a shunt is required, increase in more than 2 functional levels compared to anatomic level.
At Connecticut Children’s Fetal Care Center, we consider lateral ventricles a the time of screening >15 mm as a relative exclusion criteria based on the MOMS trial report by Tulipan and our own outcomes. In a series of 65 patients, Dr Crombleholme found that fetus’s ventricles <10 mm at screening had only a 25% rate of ventriculo-peritoneal shunt placement by one year of age, whereas those with ventricles >10 mm <15mm had a shunt rate of 37% and those with ventricles >15 mm had a 100% shunt rate. As a result we cautiously offer prenatal myelomeningomyelocele repair to carefully counseled mothers whose fetus has ventricles >15 mm at presentation as there will be no reduction in the need for ventriculo-peritoneal shunting but there may be other benefits.
The criteria used to qualify patients for this treatment option are based on our previous experience with open fetal surgery for MMC and the results of the MOMS trial. In order to be considered for prenatal MMC repair the following criteria must be met:
Inclusion Criteria
- Myelomeningocele at T1 through S1 with hindbrain herniation, level of MMC confirmed by ultrasound and hindbrain herniation confirmed by MRI.
- Lateral ventricles measure <15 mm, >15 mm cautiously offered
- Maternal age greater than or equal to 18 years.Gestational age 19 0/7 weeks to 26 0/7 at the time of prenatal surgery.
- Normal karyotype or FISH.
- Normal or minimal anomaly on fetal echocardiogram.
- Singleton pregnancy.
- Willing to remain in the greater Hartford area for at least 2 weeks after the surgery before being transferred back to the care of her referring Maternal-Fetal Medicine Specialist
Exclusion Criteria
- Ventricles >15 mm (only a relative exclusion criteria)
- Significant fetal anomaly not related to MMC.
- Kyphosis in a fetus of greater than 30 degrees.
- History of incompetent cervix, cervix less than 20mm, or presence of a cerclage.
- Morbid obesity as defined as a BMI of greater than 35.
- Maternal-fetal Rh isoimmunization, Kell sensitization, or a history of neonatal alloimmune thrombocytopenia.
- Maternal HIV, Hepatitis B, and Hepatitis C due to increased risk of transmission to the fetus during maternal–fetal surgery.
- Uterine anomalies such as large or multiple uterine fibroids or mullerian duct abnormality.
- Maternal medical condition which is a contraindication to abdominal surgery or general anesthesia.
- No support person to stay with mother at Ronald McDonald House.
- A patient does not meet psychosocial criteria as determined by the social worker evaluation.
- Previous hysterotomy in the active segment of the uterus either from previous classical cesarean section, uterine anomalies such as an arcuate or bicornuate uterus, major myomectomy resection, or previous open fetal surgery.
If a mother meets all of the qualifying criteria and wishes to proceed with prenatal surgery, she will undergo phase II counseling by a Maternal Fetal Medicine Specialist, not part of the operative team to ensure: She has been appropriately counseled about the potential obstetric, maternal and fetal risks and complications; and she has an accurate appreciation of the implications of these risks and complications prior to being consented for the surgery; a Neonatologist to consult on the implications of prematurity; a Pediatric Neurosurgeon to review the prenatal and postnatal options. A separate consent team meeting is held to review the potential anesthetic, obstetrical, neurosurgical fetal surgical, and neonatal risks of the procedure with representation of each respective discipline present to review these potential risks and complications.
The results of the MOMS trial clearly showed that prenatal repair of myelomeningocele reversed or corrected hindbrain herniation and reduced the need for ventriculoperitoneal shunting by one of age (Adzick et al 2011). Prenatal repair reduced the need for ventriculoperitoneal shunting to 40% from 82% with postnatal repair. However, the prenatal repair was associated with higher rates of obstetrical complications such as oligohydramnios, chorioamnionitis membrane separation, placental abruption, premature rupture of membranes, preterm delivery, and uterine scar dehiscence compared to postnatal repair (Adzick et al 2011, Soni et al 2016).
Fetoscopic repair of myelomeningocele was proposed to reduce these complications. Initial results reported by groups in Germany and Brazil suggested reduced rates of obstetrical complications. However, these reports also had a high rate of membrane rupture, premature birth, and a myelomeningocele closure technique that was unreliable with higher rates of CSF fluid leakage requiring postnatal revision of the myelomeningocele repair.
In a recent systematic review and meta-analysis, Kababgambe et al found that comparing fetoscopic to open fetal surgery to repair myelomeningocele had no differences in ventriculoperitoneal shunt or ventriculostomy rates at 12 months (42% for fetoscopic; 40% for open). There were no differences between fetoscopic and open techniques in a reversal of hindbrain herniation, motor response relative to anatomic level, birth <30 weeks’ gestation, chorioamniotic membrane separation, and placental abruption.
Fetoscopic myelomeningocele repair was associated with higher rates of wound closure dehiscense and/or CSF leakage requiring postnatal myelomeningocele repair site revision (30% for fetoscopic; 7% for open, p<0.01), and preterm premature rupture of membranes (79% for fetoscopic: 36% for open, p=0.04). In contrast, the rate of partial or complete uterine wound dehiscense was higher in open repair (11%) compared to fetoscopic repair (0%, p<0.01).
There are further differences in the fetoscopic techniques employed which affect the rate of complications. The German group uses an entirely percutaneous technique that does not close the trocar hysterotomy sites. In contrast, Belford et al, externalize the uterus for placement of two 4 mm trocars after securing the membranes to the uterine wall for the procedure and these trocar sites are closed at the conclusion of the procedure. This group reported that these trocar sites are well healed at the time of cesarean section delivery (Belford et al 2015).
The duration of the operative procedure is another factor for which fetoscopic surgery is significantly longer with a range of 98 to 480 minutes when performed percutaneously and 145 to 480 minutes when performed with uterine exteriorization. This is in contrast to the 34 to 130 minutes for open fetal surgical myelomeningocele repair. This has been postulated to be a contributing factor in the development of preterm premature rupture of membranes.
Pregnancy, Postnatal Care & Outcomes
Once myelomeningocele is suspected, the patient should be referred to a center capable of thorough anatomic diagnosis of the fetus. Confirmation of the MMC can be made by noting the presence of the cranial abnormalities discussed in the “Sonographic Findings” section. In addition, associated anomalies should be sought. Once the neural tube defect has been definitively identified, the parent should be offered the opportunity to obtain amniotic fluid for fetal karyotype and microarray analysis.
In a study of 77 fetuses retrospectively identified with isolated neural tube defects (Harmon et al., 1995), karyotype information was available in 43. The risk for chromosomal abnormalities based on the maternal age of this population was 0.3%. In the study group, however, 7 chromosomal abnormalities were discovered, an incidence of 16.3%. The difference between the expected occurrence of chromosomal abnormalities based on maternal age and the observed incidence of chromosomal abnormalities was highly significant. In the study, two cases of trisomy 18, three cases of triploidy, one case of a balanced Robertsonian translocation and one Xq inversion were demonstrated. Subsequent studies have confirmed that between 2% and 16% of isolated neural tube defects occur in association with a chromosome abnormality or single-gene defect (Shaer et al., 2007). The most commonly associated aneuploidy in MMC is trisomy 18. We recommend obtaining a fetal karyotype because knowledge of the fetal cytogenetic status affects prognosis, management of the pregnancy, intervention, and recurrence risks.
Once the diagnosis of neural tube defect is confirmed, the parents should be offered the opportunity to discuss the long-term prognosis for a child with MMC with pediatric subspecialists. This is best performed in the context of a multidisciplinary team. We recommend that parents meet with a neonatologist, geneticist, pediatric neurologist, pediatric neurosurgeon, pediatric urologist, pediatric orthopedic surgeon, and, if available, the physician coordinating the Spina Bifida clinic.
Long-term prognosis is related to the location of the MMC. In general, the lower the defect is on the fetus, the better the prognosis. If the diagnosis is made at <24 weeks of gestation, the parents should be made aware of the option to interrupt the pregnancy. Data from the statewide California AFP Screening Program suggest that families will act on information regarding neural tube defects. At <24 weeks of gestation, 80% of pregnant women will terminate the pregnancy when the defect is nonfatal, and 93% will terminate the pregnancy when the defect is fatal, such as anencephaly (Budorick et al., 1995).
If the diagnosis is made at >24 weeks of gestation, or if the parents elect to continue the pregnancy, the risks and benefits of elective cesarean section delivery prior to labor should be discussed. In 1991, Luthy et al. (1991) described their results of performing elective cesarean section without labor on fetuses with neural tube defects. They documented a lower risk of severe paralysis and on average, a motor function that was 3.3 spinal segments better than that expected on the basis of the anatomic level of the lesion when the affected children were 2 years of age. These authors suggested that unsplinted neural tissue and its blood supply were potentially traumatized by intrauterine pressures generated during labor. The study was criticized, however, because it was not randomized. With additional observations, recommendations for delivery include the following (Shurtleff and Lemire, 1995): elective cesarean section is indicated when the fetus demonstrates movement of the knees and ankles and an MMC sac is observed protruding dorsally beyond the plane of the infant’s back; cesarean section is contraindicated for fetuses with a known chromosomal abnormality, other congenital anomalies that significantly interfere with survival or the absence of fetal knee or ankle movement; cesarean section has not been shown to be beneficial in primiparous women with a fetus already engaged in the breech position, fetuses with gibbous deformities and fetuses with hypoplastic spinal cords.
The newborn infant with myelomeningocele should be handled in as sterile a manner as possible in a latex-free environment. The spinal lesion should be immediately covered with a nonadherent dressing moistened with warm physiologic Ringer’s lactate or normal saline. A firm, protective ring of sterile dressings should be placed around the sac and the sac itself should be covered with a nonadhesive dressing (Hahn, 1995; Shurtleff and Lemire, 1995). If the infant needs to be intubated, this should be performed in the prone or in the lateral recumbent position if possible. At all times, normothermia must be maintained.
An initial physical examination should be performed by the neonatologist and the pediatric neurosurgeon to assess the functional level and the extent of the neurologic deficit. The sensory level can be determined by stimulating dermatomes with pinpricks. The spinal column should be examined for evidence of early scoliosis or kyphosis. Consideration should be given to performing a baseline head ultrasound and an MRI scan so that the neurosurgeon can plan the postnatal surgical approach. The parents should be informed that if hydrocephalus is not present pre-operatively, it may develop after repair of the neural tube defect. Generally, if a shunt is necessary, it is placed before subsequent urologic or orthopedic repairs.
The Arnold-Chiari type II malformation is present in 95% of patients with MMC. In 6% of affected patients, central ventilatory dysfunction may be present, as demonstrated by central apnea, stridor, respiratory distress, or aspiration. Bulbar involvement may result in vocal cord paralysis or dysphagia. Unfortunately, approximately half of all newborns with MMC have pneumographic abnormalities or abnormal responses to increasing CO2 content in inspired air (Petersen et al., 1995). Therefore, standard tests of respiratory function are not useful to predict which infants will become symptomatic because of an Arnold-Chiari malformation.
The earliest recorded surgical treatment of a child with spina bifida was performed in 1910 (Hahn 1995). With the development of antibiotics, there was increased interest in treating this condition. It is currently recommended that surgical closure should occur within the first 24 to 48 hours of life to decrease morbidity and mortality.
Exposure of neural tissue to trauma during birth potentially causes a shock-like state to the neural placode. The goals of operative repair include preserving all viable neural tissue, reconstituting a normal anatomic environment, and minimizing the chance of infection or preventing ascending infection of the neural axis (Hahn 1995). During repair, the neurosurgeon must: identify the neural placode, intermediate epithelial layer, and the pia, arachnoid, and dura; preserve neural tissue; reconstitute a normal neural environment with reconstitution of the pial, arachnoidal, dural, and fascial and skin layers, complete skin closure; and prevent leakage of cerebrospinal fluid (Pang 1995) The operative mortality for myelomeningocele repair is near 0% and expected survival is 95% or higher for the first 2 years of life (Hahn 1995).
Hydrocephalus develops in 80% of cases of myelomeningocele. This is often not apparent until the neural tube defect is repaired. It is not uncommon to require a shunt placement within a few days after neural tube defect repair. There have been significant changes in the indication for ventriculo-peritoneal shunt placement over the last decade and the MOMS trial developed criteria to standardize the indications for shunt placement. These criteria were used for the MOMS trial and have been widely adapted for use in newborn nurseries to assess the need for ventriculoperitoneal shunging for myelomeningocele.
At least two of the following:
1. An increase in the greatest occipital-frontal circumference adjusted for gestational age is defined as crossing percentiles. Patients who cross centiles and subsequently plateau do not meet these criteria
2. A bulging fontanelle (defined as above the bone assessed when the baby is in an upright position and not crying) or split sutures or sunsetting sing (eyes appear to look downward with the sclera prominent over the iris)
3. Increasing hydrocephalus on consecutive imaging studies determined by the increase in the ratio of biventricular diameter to biparietal diameter according to the method of O’Hayon et al. (O’Hayon BB, Drake JM, Ossip MG, et al. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998; 29:245-9).
4. Head circumference >95th percentile for gestational age
Or
1. Presence of marked syringomyelia (syrinx with expansion of spinal cord) with ventriculomegaly (undefined).
Or
2. Ventriculomegaly (undefined) and symptoms of Chiari malformation (stridor, swallowing difficulties, apnea, bradycardia)
Or
3. Persistent cerebrospinal fluid leakage from the myelomeningocele wound or building at the repair site.
The long-term considerations for the infant and child with myelomeningocele include neuromuscular and urologic function, as well prevention of orthopedic abnormalities. To stand erect, motor function is needed to at least the third lumbar level. To walk, the child must exhibit motor function from the fourth to the fifth lumbar level. To function sexually as an adult, the individual must have motor function to at least the second to fourth sacral level.
The degree of handicap and survival rate depends on the level of spinal segments, the severity of the lesion, the treatment program, and the associated anomalies (Budorick et al. 1995). The lower the spinal level, the better the prognosis. Prediction of long-term IQ is impossible. Approximately one-fourth of patients have an IQ below 50, one-fourth of patients have an IQ above 100, and 50% of patients have a learning disability (Budorick et al. 1995).
The Arnold–Chiari malformation is the most common cause of death. Hindbrain dysfunction causes death within the first year of life in a small percentage of patients (Rauzzino and Oakes 1995). Symptomatic patients present with apnea, aspiration, swallowing difficulties, and opisthotonos. Considerations for surgical decompression of the hindbrain include:
1. Inspiratory stridor at rest.
2. Aspiration pneumonia due to palatal dysfunction or gastroesophageal reflux.
3. The presence of central apnea with or without cyanosis, especially during sleep.
4. Opisthotonos.
5. Functionally significant or progressive spasticity of the upper extremities or
truncal or appendicular ataxia (Rauzzino and Oakes 1995).
In recent years, there has been an increased appreciation of the long-term consequences of renal failure in adulthood (Zawin and Lebowitz 1992). Therefore, urologic management is more aggressive and directed toward maintaining normal renal function (Stone 1995). After the neural-tube defect has been closed and a shunt has been placed, urodynamic studies are recommended. If the patient has high pressures due to bladder–sphincter dyssynergia, a voiding cystourethrogram (VCUG) can be performed to rule out vesicoureteric reflux. For many cases, anticholinergics or smooth muscle relaxants are recommended to alter the overreactivity of the abnormal detrusor.
This treatment is meant to increase the capacity of the bladder. Prophylactic antibiotics are used to prevent urinary tract infection. Children are taught how to perform clean, intermittent bladder catheterization. This technique safely and effectively empties the bladder, preventing both upper-tract deterioration and overflow incontinence (Zawin and Lebowitz 1992). If frequent urinary tract infection is a problem, a vesicostomy can be performed, creating a fistula between the bladder and the abdominal wall. Eighty percent of children with neural-tube defects achieve continence by intermittent catheterization and the use of anticholinergic medications.
The presence of myelodysplasia can lead to musculoskeletal deformities. The goals of the orthopedic surgeon are to maintain mobile and pain-free joints, as well as to prevent decubitus ulcers in insensate limbs (Karol 1995). The spine is at risk for the subsequent development of kyphosis and scoliosis. Significant spine deformities that require surgery develop in 10% of children with lesions at the thoracic level. Scoliosis is the most commonly encountered spinal deformity, which is treated by spinal fusion. Although patients with myelomeningocele can dislocate their hips, they are rarely surgically reduced. Contractures at the hip are surgically released to allow children to fit orthoses. External tibia rotation is commonly seen at the ankles. Sixty percent of children with myelomeningocele have club feet (see Chapter 104). This is treated differently for the child with myelomeningocele as compared with otherwise normal children. Neurologic club feet are more rigid than idiopathic club feet. The application of skin casts with stretching—the usual treatment for idiopathic club foot—can lead to skin breakdown for the child with myelomeningocele. Surgical treatment is generally recommended, but this should be delayed until the child is developmentally ready to stand.
Hunt (1990) and Hunt and Poulton (1995) have described the long-term follow-up of 117 babies (50 boys, 67 girls) born between 1963 and 1971. All of these infants had surgical repair within the first 48 hours after birth. Of this cohort, 25 infants died within the first year of life, 15 died between ages 1 and 5 years, 8 died between ages 5 and 16 years, and 8 died between 16 and 25 years (Fig. 19-8). The reports cover 69 survivors in 1990 and 61 survivors in 1995. Remarkably, 16 of the 56 deaths were from renal failure. In general, survival was lowest and disability greatest when the sensory level affected was above T11 (Fig. 19-9). The survival was highest and disability the lowest when the sensory level was below L3 (Fig. 19-10). Most severely affected cases had a poor renal prognosis due to neuropathic obstruction of bladder outflow. Of the survivors in 1990, 50% could walk for 50 yards or more and 50% were wheelchair-bound. Forty-seven of the 69 survivors had an IQ of >80, 12 had an IQ of between 60 and 79, and 10 had an IQ of <60. Approximately half of the young adults remained continent or managed their own incontinence. The other half needed help from other adults, and 43% of survivors used some sort of adult diaper or padding.
With regard to disability, 12% of patients walked normally and had a normal IQ. Fifty percent of patients could walk 50 yards and had normal intelligence. Twenty-five percent of patients were severely disabled and could not walk or stand and were mentally retarded. Twelve percent of patients were very severely disabled and were blind, mentally retarded, and needed help with transfers, dressing, and incontinence.
With regard to general health, one-third of patients had precocious puberty, one-third were overweight, and visual defects were common among these patients, including blindness in two of them. The presence of a history of ventriculitis, septicemia, or intracranial hemorrhage was associated with mental retardation, epilepsy, and blindness.
Sixteen of the 69 adult survivors were employed. Their occupations included light assembly work, clerical jobs, garage mechanic, gardener, and hairdresser. These authors concluded that most of the surviving young adults were badly handicapped. Thirty percent were mentally retarded, as defined by an IQ of <80.
These authors stated that the sensory level to pinprick at birth was especially useful in the determination of long-term disability. When the sensory level was above T11, there was 50% survival to adult life. If the patient survived, there was a 50% chance of a normal IQ. Most of these patients were severely disabled, with no prospect of walking or continence and only a 10% chance of being able to live independently. When the sensory level was between T11 and L3, there was a 55% chance of survival to adulthood. If the patient survived, there was a 70% chance of a normal IQ. Of this group of patients, 40% could walk, 15% were continent, and 45% were able to live independently.
For the patients with a sensory level below L3, there was a 70% chance of survival to adulthood, and for the survivors there was an 80% chance of a normal IQ. Of these patients, 90% could walk and 45% were continent. Eighty-five percent of this group were able to live independently (Hunt 1990; Hunt and Poulton 1995). It should be noted that much as changed in the care and management of children with myelomeningocele in the last 20 years and it is likely that these numbers do not reflect current outcomes.
The vast majority of neural tube defects are isolated and multifactorial in origin (Main and Mennuti 1986). In a landmark study, Holmes et al. (1976) classified 106 stillborn and liveborn infants with neural tube defects. They identified six different causes for the anomalies. This study consisted of a retrospective analysis of 79 autopsy cases and a prospective analysis of 27 live-born infants. Of the 27 liveborn infants, 23 had an isolated neural tube defect, and of these 15 had anencephaly. Of the 79 retrospective cases, 67 were consistent with multifactorial inheritance. Five of the cases were consistent with a single-gene disorder, and all were thought to be due to Meckel–Gruber syndrome, an autosomal recessive condition. One case was clinically diagnosed as trisomy 13, although the definitive chromosome analysis was unavailable. One of the 79 was an infant of a diabetic mother. Three cases were due to amniotic bands, and 2 cases were due to cloacal exstrophy. These authors suggested that the cause of neural tube defects was highly variable and that genetic counseling could not support a uniform 5% recurrence risk for all patients.
More recently, authors have suggested a recurrence risk of 1.5 to 3%, although with two affected siblings the recurrence risk increases to 5.7% in the United States and 12% in the United Kingdom (Main and Mennuti 1986). For sisters of the mother who give birth to an affected child with a neural tube defect, there is an increased risk. The presence of spina bifida occulta of a single vertebra in a parent does not seem to increase the risk for a neural tube defect in the offspring. The recurrence risks and associated anomalies vary between closure sites, so it is hoped that in the future, more accurate genetic counseling will be given based on the location of the neural tube defect in the proband (Van Allen et al. 1993).
Women who have already given birth to an affected infant with a neural tube defect should be given 0.4 to 0.8mg of folic acid preconceptually prior to the next pregnancy (Wald et al. 1991). Several studies to date have demonstrated the preventive effect of folic acid supplementation prior to conception. These studies have been summarized by Rieder (1994). In one study, 11 cases of neural tube defects were observed in 1188 pregnancies supplemented with folic acid, or a prevalence of 0.9 per 100 live births. The patients not taking supplements had 54 cases of neural-tube defects in 1286 live births, for a prevalence of 4 cases per 100. The mechanism by which folic acid mediates neural tube closure is unknown, although the preventive effect has been shown even in low-risk women (Milunsky et al. 1996). It is currently recommended that all women of childbearing age take at least 0.4 to 0.8mg of folic acid every day (Baty et al. 1996; Wald et al. 1991).
Prenatal diagnosis of neural tube defects in a subsequent pregnancy can be performed by maternal serum AFP screening, amniotic fluid AFP screening, and prenatal sonographic examination.
Aaronson OS, Hernanz-Schulman M, Bruner J, et al. Myelomeningocele: prenatal evaluation—comparison between transabdominal US and MR imaging. Radiology. 2003;227:839-843.
Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675-1676.
Adzick NS, Walsh DS. Myelomeningocele: prenatal diagnosis, pathophysiology and management. Semin Pediatr Surg. 2003;12:168-174.
Arnold J. Transposition von Gewebskeimen und Sympodie. Beitr Pathol Anat. 1894;16:1.
Babcock CJ, Goldstein RB, Barth RA, et al. Prevalence of ventriculomegaly in association with myelomeningocele: correlation with gestational age and severity of posterior fossa deformity. Radiology. 1994;190:703-707.
Ball RH, Filly RA, Goldstein RB, Callen PW. The lemon sign: not a specific indicator of meningomyelocele. J Ultrasound Med. 1993;3:131-134.
Bannister CM, Russell SA, Rimmer S. Prenatal brain development of fetuses with myelomeningocele. Eur J Pediatr Surg. 1998;8(suppl 1):15-17.
Barry A, Patten BM, Stewart BH. Possible factors in the development of the Arnold-Chiari malformation. J Neurosurg. 1957;14:285-301.
Baty BJ, Cohen L, Phelps L, et al. Folic acid and the prevention of neural tube defects: a position paper of the National Society of Genetic Counselors. J Genet Couns. 1996;5:139-143.
Belfort MA, Whitehead WE, Shamshirsaz AA, Ruano R, Cass DL, Olutoye OO. Fetoscopic Repair of Meningomyelocele. Obstet Gynecol. 2015; 126: 881–884.
Belfort MA, Whitehead WE, Shamshirsaz AA, Espinoza J, Nassr AA, Lee TC, Olutoye OO, Keswani SG, Sanz Cortes M. Comparison of two fetoscopic open neural tube defect repair techniques: single- vs three-layer closure. Ultrasound Obstet Gynecol. 2020 Oct;56(4):532-540.
Bianchi DW, Crombleholme TM, D’Alton ME, Malone FA, second edition – Fetology: Diagnosis and Management of the Fetal Patient McGraw Hill, New York, NY. 2010
Biglan AW. Strabismus associated with myelomeningocele. I Pediatr Ophthalmol Strabismus. 1995;32:309.
Blumenfeld Z, Siegler E, Bronshtein M. The early diagnosis of neural tube defects. Prenat Diagn. 1993;13:863-871.
Boat A, Mahmoud M, Michelfelder EC, Lin E, Ngamprasertwong P, Schnell B, Kurth CD, Crombleholme TM, Sadhasivam S: Supplementing desflurane with intravenous anesthesia reduces cardiac dysfunction during open fetal surgery. Paediatr Anaesth 2010 )8) 748-756
Bouchard S, Davey MG, Rintoul NE, et al. Correction of hindbrain herniation and anatomy of the vermis following in utero repair of myelomeningocele in sheep. J Pediatr Surg. 2003;38:451.
Brock DJH, Sutcliffe RG. Alpha-fetoprotein in antenatal diagnosis of anencephaly and spina bifida. Lancet. 1972;2:197.
Brock JW 3rd, Thomas JC, Baskin LS, Zderic SA, Thom EA, Burrows PK, Lee H, Houtrow AJ, MacPherson C, Adzick NS; Eunice Kennedy Shriver NICHD MOMS Trial Group. Effect of Prenatal Repair of Myelomeningocele on Urological Outcomes at School Age. J Urol. 2019 Oct;202(4):812-818. Epub 2019 Sep 6.
Bruner JP, Tulipan NE, Richards WO. Endoscopic coverage of fetal open myelomeningocele in utero. Am J Obstet Gynecol. 1997;176:256-257.
Bruner JP, Richards WO, Tulipan NB, Arney TL. Endoscopic coverage of fetal myelomeningocele in utero. Am J Obstet Gynecol. 1999 Jan;180:153-8.
Budorick NE, Pretorius DH, Nelson TR. Sonographic of the fetal spine: technique, imaging findings, and clinical implications. AIR Am Roentgenol. 1995;164:421-428.
Caldarelli M, Di Rocco C, La Marca F. Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst. 1996;12:748-754.
Campbell J, Gilbert WM, Nicolaides KH, Campbell S. Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet Gynecol. 1987;70:247-250.
Carr MC. Bladder management for patients with myelodysplasia. Surg Clin North Am. 2006;86:515-523.
Carr MC. Fetal myelomeningocele repair: urologic aspects. Curr Opin Urol. 2007;17:257-262.
Catala M. Embryogenesis: why do we need a new explanation for the emergence of spina bifida with lipoma? Childs Nerv Syst. 1997;13:336.
Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14): 1-7.
Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate-United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53:362-365.
Chen Z, Cremer R, Baur X. Latex allergy correlates with operations. Allergy. 1997;52:873.
Chiari H. Ueber Veraenderungen des Kleinhirns der Pons und der Medulla oblongata in folge von congenitaler Hydrocephalie des Grosshirns. Dtsch Akad Wissenschr Math Natur Klin. 1895;63:71.
Cremer R, Kleine-Diepenbruck U, Hoppe A, et aL Latex allergy in spina bifida patients-prevention by primary prophylaxis. Allergy. 1998; 53:709-711.
Danzer E, Adzick S, Gerdes M, et al. Lower extremity neuromotor function following in utero myelomeningocele repair. Am J Obstet Gynecol. 2006:195;S22, abstract.
Danzer E, Johnson MP, Bebbington M, et al. Fetal head biometry assessed by fetal magnetic resonance imaging following in utero myelomeningocele repair. Fetal Diagn Ther. 2007;22:1-6.
Degenhardt J, Schürg R, Winarno A, Oehmke F, Khaleeva A, Kawecki A, Enzensberger C, Tinneberg H-R, Faas D, Ehrhardt H, Axt-Fliedner R, Kohl T. Percutaneous minimal-access fetoscopic surgery for spina bifida aperta. Part II: maternal management and outcome. Ultrasound Obstet Gynecol. 2014 Nov;44(5):525-31.
Den Ouden AL et al. Prevalenties, klinish beeld en prognose van neuralbuidefecten in Netherland. Ned Tijdschr Geneeskd. 1996;140:2092.
Dias MS. Myelomeningocele repair in utero. Pediatr Neurosurg 1999;30:108.
Goldstein RB, Podrasky AE, Filly RA, Callen PW. Effacement of the fetal cisterna magna in association with myelo-meningocele. Radiology 1989;172:409–413.
Graf K, Kohl T, Neubauer BA, Dey F, Faas D, Wanis FA, Reinges MHT, Uhl E, Kolodziej MA. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part III: neurosurgical intervention in the first postnatal year. Ultrasound Obstet Gynecol. 2016 Feb;47(2):158-61.
Holmes LB, Driscoll SG, Atkins L. Etiologic heterogeneity of neural tube defects. N Engl J Med 1976;294:365–369.
Dinh DH, Wright RM, Hanigan WC. The use of magnetic resonance imaging for the diagnosis of fetal intracranial anomalies. Childs Nerv Syst. 1990;6:212-215.
Drewek MJ, Bruner JP, Whetsell WO, et al. Quantitative analysis of the toxicity of human amniotic fluid to cultured rat spinal cord. Pediatr Neurosurg. 1997;27:190-193.
Ehlers K, Sturje H, Merker HJ, et al. Spina bifida aperta induced by valproic acid and by all- trans-retinoic acid in the mouse: distinct differences in morphology and periods of sensitivity. Teratology. 1992;46: 117-130.
Filly RA. Ultrasound evaluation of the fetal neural axis. In: Callen PW,ed. Ultrasonography in Obstetrics and Gynecology. Philadelphia: WB Saunders Co; 1994:189.
Glenn OA, Barkovich J. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis: part 2. AJNR Am J Neuroradiol. 2006;27:1807-1814.
Goldstein RB, Podrasky AE, Filly RA, Callen PW. Effacement of the fetal cisterna magna in association with myelomeningocele. Radiology. 1989;172:409-413.
Hahn YS. Open myelomeningocele. Neurosurg Clin N Am. 1995;6:231-241.
Harmon JP, Hiett AK, Palmer CG, Golichowski AM. Prenatal ultrasound detection of isolated neural tube defects: is cytogenetic evaluation warranted? Obstet GynecoL 1995;86:595-599.
Haogland MD, Chatterjee D: Anesthesia for fetal surgery. Paediatr Anaesth 2017: (4) 346-357
Hoffman HJ, Hendrick EB, Humphreys RP. Manifestations and management of Arnold Chiari malformation in patients with myelomeningocele. Childs Brain. 1975;1:255.
Holmes LB, Driscoll SG, Atkins L. Etiologic heterogeneity of neural tube defects. N Engl I Med. 1976;294:365-369.
Hunt GM, Poulton A. Open spina bifida: a complete cohort reviewed 25 years after closure. Dev Med Child Neurol. 1995;37:19-29.
Hunt GM. Open spina bifida: outcome for a complete cohort treated unselectively and followed into adulthood. Dev Med Child Neurol. 1990;32:1088-1118.
Hutchins GM, Meuli M, Meuli-Simmen C, et al. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996;16:701-712.
Iborra J, Pages E, Cuxart A. Neurological abnormalities, major orthopaedic deformities and ambulation analysis in a myelomeningocele population in Catalonia (Spain). Spinal Cord. 1999;37:351-357.
Johnson MP, Gerdes M, Rintoul N, et al. Maternal-fetal surgery for myelomeningocele: neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol. 2006;194:1145-1152.
Kabagambe SK, Jensen GW, Chen YJ, Vanover MA, Farmer DL. Fetal Surgery for Myelomeningocele: A Systematic Review and Meta-Analysis of Outcomes in Fetoscopic versus Open Repair. Fetal Diagn Ther. 2018;43(3):161-174.
Kallen B, Cocchi G, Knudsen LB, et aL International study of sex ratio and twinning of neural tube defects. Teratology. 1994;50:322-331.
Karol LA. Orthopedic management in myelomeningocele. Neurosurg Clin N Am. 1995;6:259-268.
Kohl T. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part I: surgical technique and perioperative outcome. Ultrasound Obstet Gynecol. 2014 Nov;44(5):515-24.
Kohn JR, Rao V, Sellner AA, Sharhan D, Espinoza J, Shamshirsaz AA, Whitehead WE, Belfort MA, Sanz Cortes M. Management of Labor and Delivery After Fetoscopic Repair of an Open Neural Tube Defect. Obstet Gynecol. 2018 Jun;131(6):1062-1068
Kollias SS, Goldstein RB, Cogen PH, Filly RA. Prenatally detected myelomeningoceles: sonographic accuracy in estimation of the spinal level. Radiology. 1992;185:109-112.
Korenromp MJ, van Gool JD, Bruinese HW, et al. Early fetal leg movements in myelomeningocele. Lancet. 1986;1:917-918.
Kreder KJ, Young PR, Worley G, Webster GD. Anomalies associated with myelodysplasia. Urology 1992;34:248–250.
Levine D, Barnes PD, Madsen JR, et al. Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstetr Gynecol. 1999;94:1011-1019.
Lichtenstein BW. Distant neuroanatomic complications of spina bifida (spinal dysraphism), hydrocephalus, Arnold-Chiari deformity, stenosis of the aqueduct of Sylvius, etc., pathogenesis and pathology. Arch Neurol Psychiatry. 1942;47:195.
Luthy DA, Wardinsky T, Shurtleff DB, et al. Cesarean section before the onset of labor and subsequent motor function in infants with meningomyelocele diagnosed antenatally. N Eng I Med. 1991;324:662-666.
Main DM, Mennuti MT. Neural tube defects: issues in prenatal diagnosis and counseling. Obstet Gynecol. 1986;67:1-16.
Matthews TJ, Honein MA, Erickson JD. Spina bifida and anencephaly prevalence-United States, 1991-2001. MMWR Recomm Rep. 2002;51(RR-13):9-11.
McComb JG. Spinal and cranial neural tube defects. Semin Pediatr Neurol. 1997;4:156.
McComb JG, Chen TC. Closed spinal neural tube defects. In: Tindall GT, Cooper PR, Barrow DL, eds. The Practice of Neurosurgery. Baltimore, MD: Williams & Wilkens; 1996:2753.
McLone DG. Results of treatment of children born with a myelomeningocele. Clin Neurosurg. 1983;30;407.
McLone DG. Care of the neonate with myelomeningocele (abstract). Neurosurg Gun N Am. 1998;9:111.
McLone DG, Diaz L, Kaplan WE, Sommers MW. Concepts in the management of spina bifida. In: Humphreys RE, ed. Concepts in Pediatric Neurosurgery. Basel, Switzerland: Karger; 1985:97.
McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15:1.
McLone DG, Naidich TP. Myelomeningocele: outcome and late complications. In:
Mclaurin RL, Schut L, Venes JL, Epstein F, eds. Pediatric Neurosurgery. Philadelphia: WB Saunders Co; 1989.
Meuli M, Meuli-Simmen C, Hutchins GM, Seller MJ, Harrison MR, Adzick NS. The spinal cord lesion in human fetuses with myelomeningocele: implications for fetal surgery. J Pediatr Surg. 1997;32:448-452.
Meuli-Simmen C, Meuli M, Hutchins GM, et aL Fetal reconstructive surgery: experimental use of the latissimus dorsi flap to correct myelomeningocele in utero. Plastic Reconstructive Surgery. 1995;96:1007-1011.
Milunsky A. Congenital defects, folic acid, and homeo-box genes. Lancet. 1996;348:419-420.
Moore CA, Li S, Li Z, et al. Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am J Med Genet. 1997;73:113-118.
Myrianthopoulos MC, Melnick M. Studies in neural tube defects. I. Epidemiologic and etiologic aspects. Am J Med Genet. 1987;26:783-796.
Neumann PE, Frankel WN, Letts VA, et al. Multifactorial inheritance of neural tube defects: localization of the major gene and recognition of modifiers in ct mutant mice. Nat Genet. 1994;6:357-362.
Nicolaides KH, Campbell S, Gabbe SG, Guidetti R. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet. 1986;2:72-74.
O’Rahilly R, Muller F. The two sites of fusion of neural folds and the two neuropores in the human embryo. Teratology. 2002;65:162-170.
Padget DH. Spina bifida embryonic neuroschisis – a causal relationship; definition of the postnatal confirmations involving a bifid spine. Johns Hopkins Med J. 1968;128:233.
Paek BW, Farmer DL, Wilkinson CC, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183:1119.
Pang D. Surgical complications of open spinal dysraphism. Neurosurg Gun N Am. 1995;6:243-257.
Patel SK, Habli MA, McKinney DN, Tabbah SM, Lim FY, Peiro JL, Stevenson CB. Fetoscopic multilayer, dural patch closure technique for intrauterine myelomeningocele repair: 2-dimensional operative video. Oper Neurosurg. 2020 Oct 12;opaa309.
Patten B. Embryological stages in the establishing of myeloschisis with spina bifida. Am J Anat. 1953;93:365-395.
Peadar KN, Mills JL, Brody LC, et al. Impact of the MTHFR C677T polymorphism on risk of neural tube defects: case control study. BMJ. 2004;328:1534-1536.
Pedreira DA, Zanon N, Nishikuni K, Moreira de Sá RA, Acacio GL, Chmait RH, Kontopoulos EV, Quintero RA: Endoscopic surgery for the antenatal treatment of myelomeningocele: the CECAM trial. Am J Obstet Gynecol. 2016; 214: 111.e1–111.e11.
Penfield W, Coburn DF. Arnold-Chiari malformation and its operative treatment. Arch Neurol Psychiatry. 1938;40:328.
Petersen MC, Wolraich M, Sherbondy A, Wagener J. Abnormalities in control of ventilation in newborn infants with myelomeningocele. J Pediatr. 1995;126:1011-1015.
Ramin KD, Raffel C, Bredde RJ, et al. Chronology of neurological manifestations of prenatally diagnosed open neural tube defects. J Matern Fetal Neonatal Med. 2002;11:89-92.
Rauzzino M, Oakes WJ. Chiari II malformation and syringomyelia. Neurosurg Clin N Am. 1995;6:293-307.
Rieder MJ. Prevention of neural tube defects with periconceptional folic acid. Clin Perinatol. 1994;21:483-503.
Rihs HP, Cremer R, Chen Z, et al. Molecular analysis of DRB and DQB1 alleles in German spina bifida patients with and without IgE responsiveness to the latex major allergen Hey b 1. Clin Exp Allergy. 1998;28:175-180.
Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109:409-413.
Ruge JR, Masciopinto J, Storrs BB, et al. Anatomical progression of the Chiari II malformation. Childs Nerv Syst. 1992;8:86-91.
Sanz Cortes M, Lapa DA, Acacio GL, Belfort M, Carreras E, Maiz N, Peiro JL, Lim FY, Miller J, Baschat A, Sepulveda G, Davila I, Gielchinsky Y, Benifla M, Stirnemann J, Ville Y, Yamamoto M, Figueroa H, Simpson L, Nicolaides KH. Proceedings of the First Annual Meeting of the International Fetoscopic Myelomeningocele Repair Consortium. Ultrasound Obstet Gynecol. 2019; 53: 855–863.
Seller MJ. Further evidence for an intermittent pattern of neural tube closure in humans. J Med Genet 1995;32: 205–207.
Shaer CM, Chescheir N, Schulkin J. Myelomeningocele: a review of the epidemiology, genetics, risk factors for conception, prenatal diagnosis, and prognosis for affected individuals. Obstet Gynecol Sun/. 2007; 62:471-479.
Shaw GM, Jensvold NG, Wasserman CR, Lammer EL Epidemiologic characteristics of phenotypically distinct neural tube defects among 0.7 million California births 1983-1987. Teratology. 1994;49:143-149.
Shaw GM, Todoroff K, Finnell RH, et al. Spina bifida phenotypes in infants or fetuses of obese mothers. Teratology. 2000;61:376-381.
Sherman MS, Kaplan JM, Effgen S, et al. Pulmonary dysfunction and reduced exercise capacity in patients with myelomeningocele. J Pediatr. 1997;131:413-418.
Shurtleff DB, Lemire RJ. Epidemiology, etiologic factors, and prenatal diagnosis of open spinal dysraphism. Neurosurg Clin N Am. 1995;6:183-193.
Shurtleff DB, Luthy DA, Nyberg DA, et al. Meningomyelocele: management in utero and post natum. Ciba Found Symp. 1994;181:270.
Sival DA, Begeer JH, Staal-Schreinemachers AL, et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum Dev. 1997;50:27-37.
Stone AR. Neurologic evaluation and urologic management of spinal dysraphism. Neurosurg Clin N Am. 1995;6:269-277.
Sutton LN, Adzick NS, Bilaniuk LT, et aL Improvement in hindbrain herniation by serial fetal MRI following fetal surgery for myelomeningocele. JAMA. 1999;282:1826-1831.
Szépfalusi Z, Seidl R, Bernert G, Dietrich W, Spitzauer S, Urbanek R. Latex sensitization in spina bifida appears disease-associated. J Pediatr. 1999;134:344.
Thévenet A, Sengel P. Naturally occurring wounds and wound healing in chick embryo wings. Roux Arch Dev Biol. 1986;195:345.
Thiagarajah S, Henke J, Hogge WA, et al. Early diagnosis of spina bifida: the value of cranial ultrasound markers. Obstet Gynecol. 1990;76:54-57.
Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42:471-491.
Tsai PY, Yang TF, Chan RC, et aL Functional investigation in children with spina bifida-measured by the Pediatric Evaluation of Disability Inventory (PEDI). Childs Nerv Syst. 2002;18:48-53.
Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg. 1998;28:177-180.
Tulipan N, Hernanz-Schulman M, Bruner JP. Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr Neurosurg. 1998;29:274-278.
Tulp NP. Observationes Medicae. 5th ed. Leiden, Netherlands: Lodewijk Elzevir; 1716. Van Den Hof MC, Nicolaides KH, Campbell J, Campbell S. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol. 1990;162:322-327.
Van Der Linden IJ, Den Heijer M, Alman LA, et al. The methionine synthase reductase 66A to G polymorphism is a maternal risk factor for spina bifida. J Mol Med. 2006;84:1047-1054.
Van Allen MI, Kalousek DK, Chernoff GF, et al. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet 1993;47:723–743.
Van den Hof MC, Nicolaides KH, Campbell J, Campbell S. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol 1990;162:322–327.
Waitzman NJ, Scheffler RM, Romano PS. An assessment of total costs and policy implications. In: Waitzman NJ, Scheffler RM, Romano PS, eds. The Cost of Birth Defects: Estimates of the Value of Prevention. Lanham, MD: University Press of America; 1996:145.
Wald N, Sneddon J, Densem J, Frost C, Stone R. Prevention of neural tube defects: results of the medical research council vitamin study. Lancet. 1991;338:131-137.
Watkins ML, Rasmussen SA, Honein MA, et al. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152-1158.
Watson WJ, Chescheir NC, Katz VL, Seeds JW. The role of ultrasound in evaluation of patients with elevated maternal serum alphafetoprotein: a review. Obstet Gynecol. 1991;78:123-127.
Wood C
Worley G, Schuster JM, Oakes WJ. Survival at 5 years of a cohort of newborn infants with myelomeningocele. Dev Med Child Neurol. 1996; 38:816-822.
Zaretsky M, Liechty K, Galan HL, Behrendt NJ, Reeves S, Marwan AI, Wilkinson C, Handler M, Legueux M, Crombleholme TM: Modified hysterotomy closure technique for open fetal surgery. Fet Diagn Ther 2017 42:28-34
Zawin JK, Lebowitz RL. Neurogenic dysfunction of the bladder in infants and children: recent advances and the role of radiology. Radiology. 1992;182:297-304.