Congenital diaphragmatic hernia (CDH) is among the most challenging anomalies to manage in the neonatal intensive care unit. The reason for this difficulty is primarily due to the pulmonary hypertension associated with CDH. We have demonstrated that having a dedicated CDH Team can dramatically improve survival and reduce the need for extracorporeal membrane oxygenation support. The CDH Team is involved in the management of the baby with CDH from the time of prenatal diagnosis, and delivery, throughout the NICU stay, and long-term follow-up. The CDH Team is composed of specialists in Maternal Fetal Medicine, Fetal Surgery, Neonatology, Pediatric Surgery, Fetal Radiology, and Fetal and Pediatric Cardiology.
A mother carrying a baby with CDH, first meets the CDH Team during her initial comprehensive prenatal CDH assessment. This assessment utilizes prognostic variables obtained from fetal MRI, ultrasound, and fetal echocardiography to develop a CDH composite prognostic index or CDH-CPI. The CDH-CPI predicts the severity of the CDH, the severity of associated pulmonary hypertension, and the impact of any associated anomalies on the outcome by integrating multiple prognostic variables to predict anticipated survival, the likelihood of the need for ECMO support, and estimate the length of stay in the NICU.
Would you like to schedule an appointment with our Fetal Care Center?
The diagnosis of CDH is usually made by routine ultrasound when the fetal stomach bubble is noted to be adjacent to the fetal heart in the case of left-sided CDH. Right-sided CDH may be more difficult to recognize as the liver is usually herniated and has an echotexture similar to that of the adjacent fetal lung.
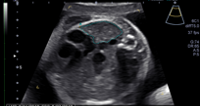
Ultrasound is an important imaging modality not only to diagnose the CDH but also to detect other anomalies which might be present, such as genitourinary, gastrointestinal, abdominal wall, and intracranial abnormalities. Important prognostic criteria are also obtained, including the lung-to-head circumference ratio (LHR) and observed-to-expected LHR. Fetal biometry is also obtained to assess fetal growth and Doppler velocimetry of the umbilical artery, umbilical vein, ductus venosus, and the middle cerebral artery.
Fetal MRI is invaluable in confirming the presence of CDH, determining the presence and degree of liver herniation, and providing multiple lung measurements that provide prognostic information. In CDH, genetic abnormalities are present in up to 20% of cases and have a disproportionate impact on survival and other outcomes. We always recommend that mothers have a genetic amniocentesis for karyotype analysis and microarray analysis. This may also have implications for the mother’s delivery. In cases in which there may be lethal anomalies, urgent cesarean delivery for fetal distress would be contraindicated as it would not benefit the baby and expose the mother to unnecessary risk. Fetal MRI is an essential tool in evaluating a baby with CDH. Fetal MRI provides exquisite anatomic detail of the hernia and better defines the degree of liver herniation. In addition, fetal MRI is used to measure total lung volume (TLV), observed to expected total lung volume (O/E TLV), and percent predicted lung volume (PPLV).
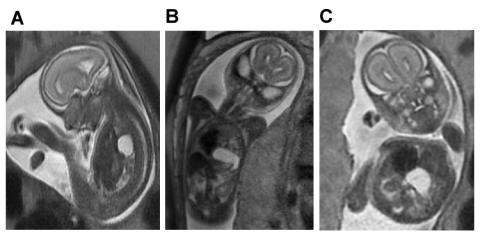
A. Saggital view of severe left-sided CDH demonstrating herniation of the left lobe of the liver filling the anterior chest and displacing the fluid filled stomach posteriorly.
B. Coronal view of the same fetus demonstrating the fluid-filled stomach rotated 180-degrees counterclockwise, sitting in the chest next to the heart.
C. Axial view of the same fetus demonstrating the relative positions of the shifted heart, herniated liver and stomach.
Fetal echocardiography is essential to rule out congenital heart defects, which may be present up to 20% of cases of CDH. In many instances, the presence of congenital heart defects may not alter the overall prognosis, survival, or long-term outcomes. However there are some critical structural heart defects, which may adversely affect outcomes due to the need for intervention to achieve adequate systemic or pulmonary blood flow, for example hypoplastic left heart syndrome. Other forms of congenital heart disease, such as a large unrestrictive ventricular septal defect may compound the risk of pulmonary hypertension. In addition to determining structural heart disease, fetal echocardiography is used to identify left ventricle to right ventricle disproportion as a marker of severity, rule out arrhythmias, and assess the severity of pulmonary hypertension.
We use the McGoon index and the prenatal pulmonary hypertension index (PPHI= the diameter of the left main pulmonary artery (in left CDH) divided by the craniocaudal length of the cerebellar vermis) to assess the severity of pulmonary hypertension. In the McGoon index, the sum of the diameters of the branch pulmonary arteries divided by the aortic diameter at the level of the diaphragm yields a ratio that is prognostically important. A McGoon index of less than 0.9 indicates the baby will have severe pulmonary hypertension and will be have pulmonary pressures equal to or greater than systemic pressures at 3 weeks of age. In contrast, when the McGoon index is greater than 1.2 the baby is likely to have less severe pulmonary hypertension with pulmonary pressure is significantly less than systemic pressure by 3 weeks of age. In the PPHI, a ratio of < 1.2 indicates the baby will have severe pulmonary hypertension with pulmonary pressures equal to or exceeding systemic pressures at 3 weeks of age. In contrast, a PPHI of > 1.5 indicates the baby’s pulmonary pressures will be significantly less than systemic by three weeks of age.
All of the data obtained during the comprehensive prenatal evaluation is integrated into a prognostic profile for the baby, which predicts the likely percent survival, the need for ECMO support, the severity of pulmonary hypertension and duration of NICU stay. The CDH Team synthesizes these data in order to outline a plan for the remainder of the pregnancy, the delivery, and postnatal management.
In those fetuses with left sided CDH with an O/E LHR of <30% or right sided CDH with O/E LHR <45% may be candidates to be treated in utero with fetoscopic endoluminal balloon tracheal occlusion or FETO. FETO has been shown to significantly improve the survival of fetuses with severe left CDH by accelerating lung growth.
The fetal lungs continue to grow throughout gestation and studies have shown that reassessment at approximately 34 weeks gestation provides an even more accurate prognostic profile. The same evaluation using ultrasound, fetal echocardiography and fetal MRI is used with an important addition: the maternal hyperoxia stimulation test. In normal developing fetal lungs, at 34 weeks gestation, the pulmonary arteries become responsive to increases in fetal blood oxygenation causing vasodilation. In the maternal hyperoxia stimulation test, a baseline McGoon index and Pulsatility Index (PI = peak systolic flow velocity minus the minimum diastolic flow velocity divided by the mean flow velocity) of the left and right main pulmonary arteries are obtained and then the mother is placed on oxygen (100% FiO2) by a non-rebreather facemask for 10 minutes.
The McGoon and Pulsatility Index are re-measured and an increase of 15% in the McGoon or 15% decrease in the Pulsatility Index of both lungs combined from baseline is considered a positive response, which indicates a normal physiologic response to oxygen and that the pulmonary hypertension will not be severe. Cases that do not meet the 15% threshold for improved McGoon or 15% decrease in PI are considered non-responders. One caveat to this is in cases in which the contralateral lung has more than 15% improvement while the ipsilateral lung does not respond to oxygen. This is a more favorable case than a non-responder as it indicates the larger, less hypoplastic lung, is able to respond to oxygen and pulmonary hypertension will not be as severe.
The CDH Team will synthesize these data at the late gestation reassessment. In 30% of cases, the CDH-CPI index will be significantly different from the first CDH-CPI obtained at presentation. In these cases, half get worse and half get better. We believe this is due to the greater herniation in the former, which prevents lung growth, and interval lung growth and development in the latter.
In CDH, even if it is an isolated defect, there is a 10% rate of intrauterine fetal demise, a term used when a fetus dies after the 20th week of gestation. The cause of these losses is unknown but tend to occur in late gestation and for that reason, we recommend delivery at 38 weeks gestation. We recommend twice-weekly antenatal testing with non-stress test NST and biophysical profiles beginning at 30 weeks gestation. This way, if monitoring is non-reassuring, the mother can be admitted to labor and delivery for continuous fetal monitoring and delivery, if indicated, for fetal distress. There is no benefit to Cesarean delivery in CDH and we reserve this for obstetric indications. Induction of labor at 38 weeks gestation is planned for those mothers who can deliver vaginally. For those mothers with prior Cesarean deliveries, a repeat Cesarean delivery is scheduled at 38 weeks gestation.
A member of the CDH team is present for all CDH deliveries to assist the delivery team from the NICU. The baby is immediately intubated to prevent crying and air swallowing, which will distend the loops of the bowel in the chest. A nasogastric tube is placed to decompress the GI tract. A peripheral IV is started to administer sedation, and a chest x-ray is obtained to confirm the endotracheal tube position. The baby is then transported to the NICU for umbilical artery and venous line placement. Infusions of fentanyl or morphine sulfate and low-dose Prostin are started. An echocardiogram is obtained at this point to assess cardiac function, estimate pulmonary pressures, and the size and directional flow of shunts at the ductus arteriosus and the atrial septum. As long as there is no left ventricular dysfunction, inhaled nitric oxide (iNO) is started at 20 ppm. In addition to inhaled nitric oxide, if there is any degree of right ventricular dilation and dysfunction, IV sildenafil and milrinone infusions are started. It is important that total IV fluids, including all drips, be restricted to < 80 mL/kg/day. If blood pressure is low, we preferentially treat this with pressors such as epinephrine infusion and/or vasopressin if refractory. If pressors are needed to maintain blood pressure, the baby is presumed to be relatively adrenally insufficient and a cortisol level is drawn while starting stress dosing of steroids.
The goal of CDH management in the first hours and days of life is to prevent an acute pulmonary hypertensive crisis by making sure the baby is protected from all noxious stimuli including pain, loud noises, and bright lights. IV sedation is used to blunt the response the baby may have to routine care and IV boluses are used to pretreat the baby prior to interactions with nursing and physician staff necessary for care. A baseline head ultrasound is obtained in the first day of life should there be the need for ECMO support.
Prenatal CDH-CPI helps guide the anticipated timing of CDH repair. We base the decision on when to repair the diaphragmatic defect on when the pulmonary pressures fall to < 80% of systemic pressures based on echocardiographic estimation. In mild cases of CDH, this level is usually reached by the end of the first week of life. In moderately severe cases it may take about 2 weeks to reach this level. In severe cases, it may take more than 3 weeks to reach this level, and we have waited up to 2 months to repair the defect.
The advantage of using echocardiographic criteria for the timing of repair is that it statistically significantly improves survival compared to approaches that do not take pulmonary pressure estimates into consideration. An important caveat to this approach is in babies with large intracardiac defects, such as atrioventricular canal defects and large ventricular septal defects, in which the estimate of pulmonary pressures do not fall because the communication between the left and right sides of the heart result in systemic pressure being measured on both sides. In these cases, the prenatal CDH-CPI is helpful in planning the timing of the repair of the CDH. This approach has resulted in an overall survival of 90% in CDH, regardless of severity, and has significantly reduced the need for ECMO support.
In less severe cases of CDH, usually without liver herniation, there may be sufficient diaphragm to perform a primary repair of the defect by sewing diaphragm to diaphragm to close the defect. In more severe cases, with larger defects and more visceral herniation, particularly involving the liver, a patch will be required to close the defect. The most common material used to close diaphragmatic defects is Gore-Tex (polytetrafluoroethylene PTFE). However Gore-Tex is inelastic, so it does not grow as the baby grows, which leads to a higher rate of recurrent herniation (4 to 50%). In contrast, the use of the transverse abdominis muscle flap has a very low rate of recurrent herniation. In my experience with over 500 CDH cases, there have been only 3 cases of re-herniation (0.4%). In addition, because of the transverse abdominis muscle flap is living muscle tissue, if there is an infection, antibiotics will clear the infection. In contrast, an infected Gore-Tex patch often requires patch removal and replacement once the infection has been cleared.
Once the CDH has been repaired, the baby enters a more stable phase of care, and the focus shifts to optimizing nutrition and weaning medications no longer needed. We routinely place nasal intestinal tubes at the time of the CDH repair to allow enteral feeding, preferably with the mother’s expressed breastmilk, beginning on post-op day 1, assuming pressors are no longer in use. The management of pulmonary hypertension shifts from inhaled nitric oxide, IV sildenafil, milrinone and Prostin to enteral medications. Prostin is turned off when pulmonary pressures have reached < 80% of systemic pressures. The milrinone is weaned off once ventricular function on an echocardiogram has normalized and N-terminal pro-brain natriuretic levels have fallen to less than 100 pg/mL. Sildenafil is transitioned from IV to enteral administration. If echocardiographic estimates of pulmonary pressures remain close to systemic, the endothelin receptor blocking agent Bosentan is added, which requires liver function test monitoring on an ongoing basis.
In most instances, the severity of pulmonary hypertension CDH gradually improves as the baby’s condition becomes more stable and the lung is allowed to expand fully after repair of the CDH. In some cases, the pulmonary hypertension remains persistently severe and refractory to medical management. This sometimes delays the repair of the CDH as the pulmonary pressures remain greater than 80% systemic. In such cases, we search for other factors, which can exacerbate the pulmonary hypertension including tracheobronchial malacia, and kinking of either the distal airway or the pulmonary veins. In approximately 5 to 10% of cases of CDH, there can be tracheobronchomalacia, or softening of the trachea and/or tracheobronchial cartilage, that normally stent the airway open. This is thought to be due to compression of the developing airway by the herniated viscera (liver, stomach, spleen, and small and large intestines). The softening of the cartilage means the trachea and the mainstem bronchi collapse with aspiration. This usually does not become apparent until the baby is able to generate increased inspiratory pressures and is either extubated or is in the process of being weaned from respiratory support.
The diagnosis of tracheobronchomalacia can be made by flexible bronchoscopy via the endotracheal tube. It may be difficult, however, to quantify the amount of positive end-expiratory pressure (PEEP) to adequately stent the airway open and a “PEEP grid” may be helpful in optimizing PEEP to stent the airway and allow pulmonary hypertension to improve. A PEEP grid is obtained by determining at which PEEP returned expiratory tidal volume and compliance is optimal by measuring the PEEP at a range of levels, usually from 6 to 12 cm H2O. As long as the airway is collapsing, the pulmonary hypertension will persist and will not respond to medical treatment
In some cases of CDH, the severity of the diaphragmatic hernia results in kinking of either the mainstem bronchus or the pulmonary vein, which prevents pulmonary hypertension from improving. Bronchial kinking results in persistently atelectatic lung, which escalates pulmonary hypertension by causing reflex vasoconstriction of the pulmonary artery in that segment of the lung. This will not improve until the CDH is repaired and the compression of the airway is relieved. The kinking of the bronchus can be confirmed by bedside flexible bronchoscopy.
In large CDHs, there can also be kinking of the pulmonary veins, which may be difficult to diagnose by echocardiography alone and may necessitate cardiac catheterization. Cardiac catheterization distinguishes intrinsic pulmonary venous stenosis from kinking of the pulmonary veins by compression by herniated viscera . This is an important diagnosis to make because pulmonary hypertension will persist until it is corrected and immediate repair is indicated. Cardiac catheterization may also be indicated when the cause of persistently severe pulmonary hypertension is uncertain and after repair of the CDH and other causes have been ruled out. Not only does cardiac catheterization provide a direct measurement of the pulmonary pressures, but it can also quantitate blood flow to each lung and assess the severity of pulmonary arterial hypoplasia, rule out the presence of arteriovenous connections responsible for persistent shunting, and test the baby’s response to various therapeutic agents in the catheterization laboratory.

The baby is admitted to the NICU on the Neonatology service with the CDH Team consulting. While the Neontalogy attending will rotate during the hospitalization, the CDH Team remains consistent. A member the CDH team is on call 24/7 to address any issues with the baby. A member of the CDH Team rounds every morning with the primary team and guides the management of the baby with CDH. The CDH team also provides long-term follow-up care for all CDH babies. Parents are encouraged to join the team on morning rounds to hear updates and have any questions they may have addressed.